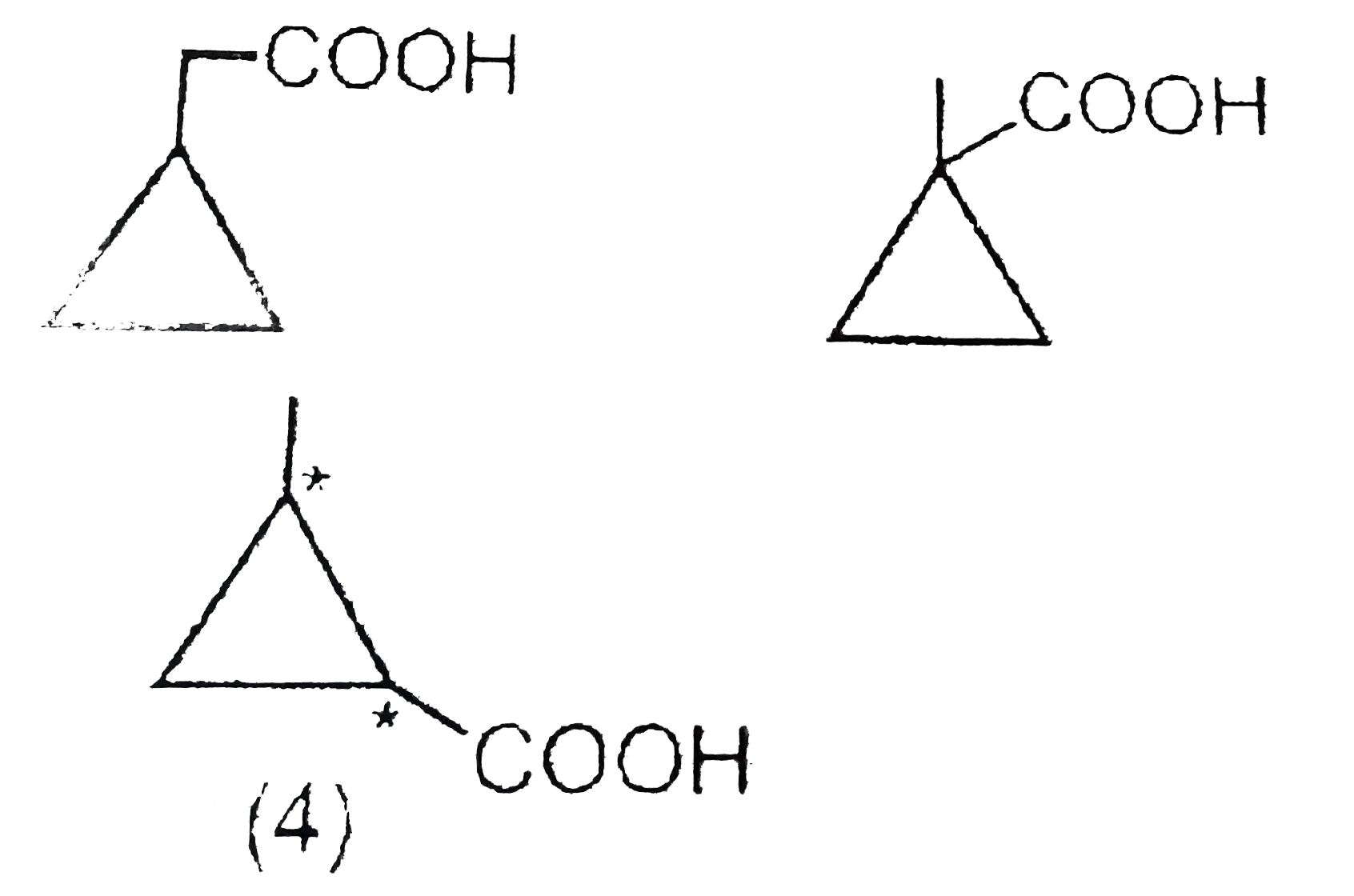Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-Chemistry
- What is sum of oxidation number and coordination number of Al in Cryol...
Text Solution
|
- What is atomicity of gaseous compound formed in the Mond's process whe...
Text Solution
|
- How many monocarboxylic acids (indluding stereo) would give Methylcycl...
Text Solution
|
- When is mixed and reacted with (Br(2))/(KOH) then how many products a...
Text Solution
|
- How many electron in K (Z =19) have n = 2, l = 1 ?
Text Solution
|
- How many of the following compounds are more reactive than benzene tow...
Text Solution
|
- In a diatomic molecule the bond distance is 1 xx 10^(-8) cm. Its dipol...
Text Solution
|
- Two types FXF angles are presnet in which of the following molecule (X...
Text Solution
|
- Which one of the following compounds is a peroxide ?
Text Solution
|
- Which of the following is thermally stable carbonate?
Text Solution
|
- Which of the following statements is correct with respect to the metal...
Text Solution
|
- The electrode potentials for Cu^(2+) (aq) +e^(-) rarr Cu^+ (aq) ...
Text Solution
|
- During electrolysis of an aqueous solution of CuSO(4) using copper ele...
Text Solution
|
- Densities of diamond and graphite are 3.5g//mL and 2.3g//mL Delta(r)H...
Text Solution
|
- How many electron in K (Z =19) have n = 3, l = 1 ?
Text Solution
|
- At least how many half-lives should elapse for a 1^(st) order reaction...
Text Solution
|
- Which of the following does not represent a first order reation?
Text Solution
|
- How many electron in Na (Z =11) have n = 2, l = 0 ?
Text Solution
|
- How many electron in Na (Z =11) have n = 3, l = 0 ?
Text Solution
|
- dz^(2) orbital has:
Text Solution
|