A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-Chemistry
- Which of the following is/are correct statement(s)?
Text Solution
|
- Select the correct statement(s)
Text Solution
|
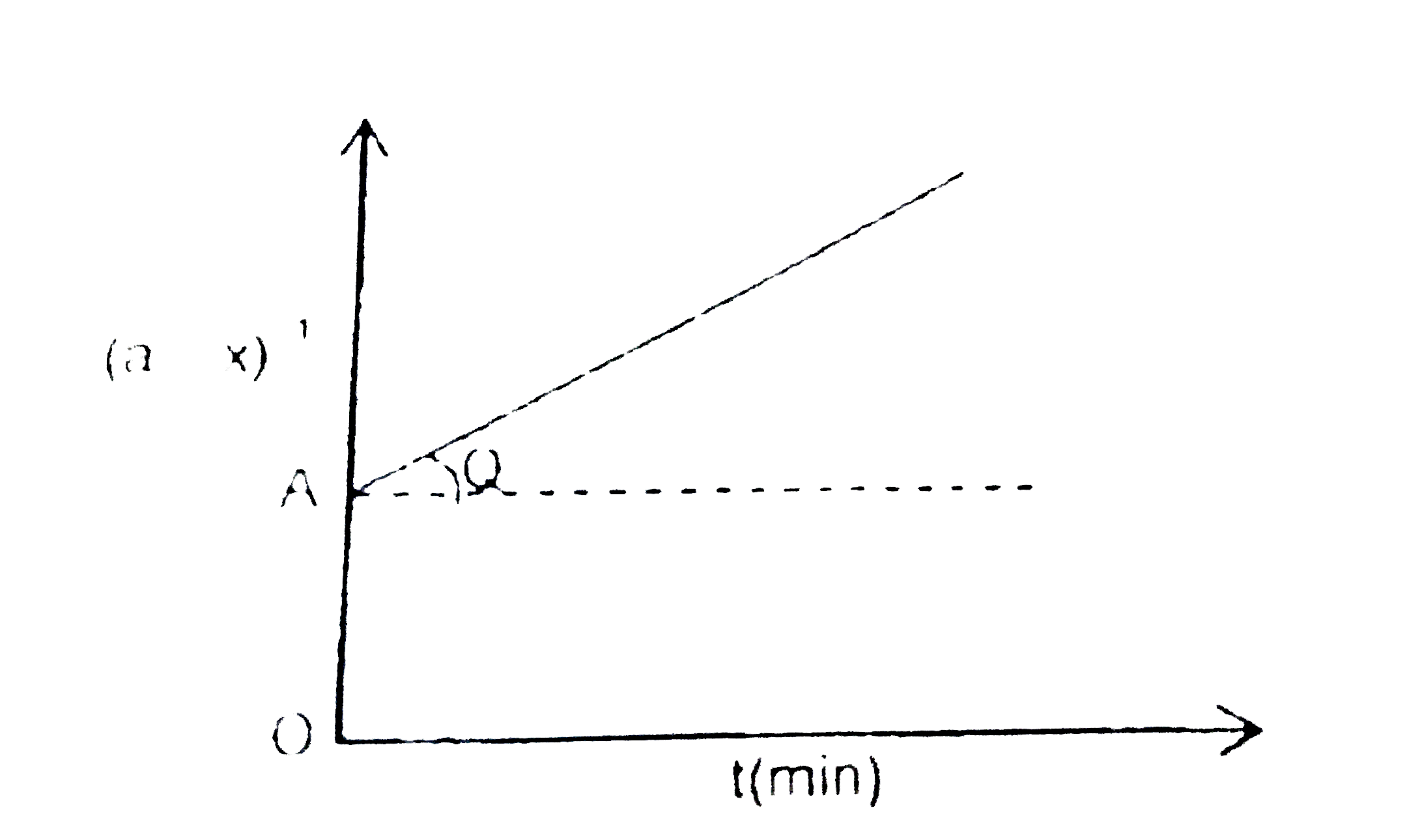
- Following is the graph between (a-x)^(-1) and time for second order re...
Text Solution
|
- The reagent that distinguishes between silver and lead salt is
Text Solution
|
- Which of the following statements(s) is/are true for XeF(6)?
Text Solution
|
- Rate constant of a reaction (k) is 4 xx 10^(-2) "litre"^(2) "mol"^(-2)...
Text Solution
|
- Select the correct statement(s)
Text Solution
|
- alpha -maltose can be hydrolysed to glucose according to the following...
Text Solution
|
- The xenate ion (HXeO(4)^(-)) undergoes disproportionation reaction to ...
Text Solution
|
- Which of the following is/are correct name for the give polymer?
Text Solution
|
- Select the correct statement(s)
Text Solution
|
- Rate constant of a reaction (k) is 4 xx 10^(-2) "litre"^(-1) molsec^(-...
Text Solution
|
- Rate constant of a reaction (k) is 2 xx 10^(-3) "litre"^(-1) molsec^(-...
Text Solution
|
- Rate constant of a reaction (k) is 3 xx 10^(-2) "litre"^(-1) molsec^(-...
Text Solution
|
- In an atom with atomic no. 35 and mass no. 85,the number of electron i...
Text Solution
|
- Two ions A^(o+) and B^(Θ) have radii 88 and 200 pm, respectively. In t...
Text Solution
|
- Let the plasma cell wall of shark is a semipermeable membrane. When th...
Text Solution
|
- 2SO(2)(g)+O(2)(g)hArr2SO(3)(g) Starting with 2 mol of SO(2) and 1 m...
Text Solution
|
- How many uncharged resosnace structures are possible for given azulene...
Text Solution
|
- The de Broglie wavelength of a particle of mass 2 gram and velocity 10...
Text Solution
|