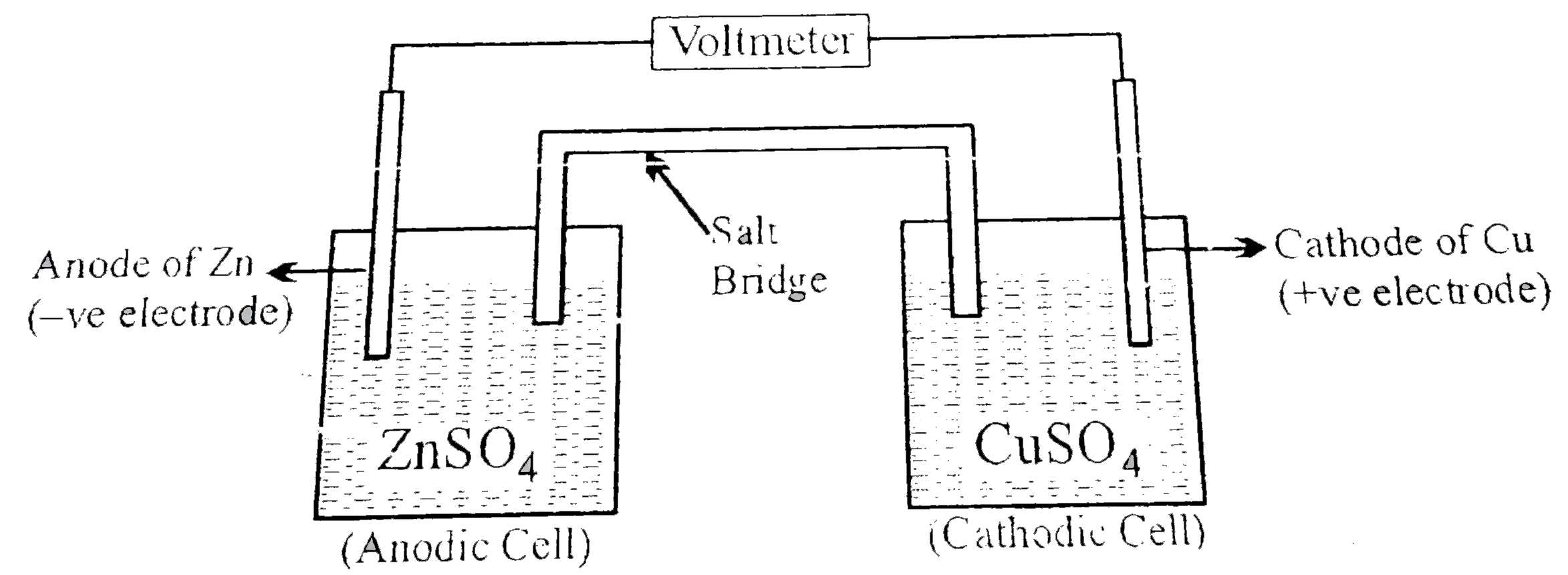A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-Chemistry
- Select correct statement(s)
Text Solution
|
- Which of the following is/are incorrect statement regarding glucose:
Text Solution
|
- The most pupular electrochemical cell, daniel cell was originally deve...
Text Solution
|
- The most pupular electrochemical cell, daniel cell was originally deve...
Text Solution
|
- (i) P+C("carbon")+Cl(2) to Q+CO uaarr (ii) Q+H(2)O to R+HCl (iii) ...
Text Solution
|
- (i) P+C("carbon")+Cl(2) to Q+CO uaarr (ii) Q+H(2)O to R+HCl (iii) ...
Text Solution
|
- The de Broglie wavelength of a particle of mass 3 gram and velocity 20...
Text Solution
|
- The de Broglie wavelength of a particle of mass 2 gram and velocity 30...
Text Solution
|
- In an experiment, 50 mL of 0.1 M solution off a metallic salt M(NO(3))...
Text Solution
|
- In the decomposition of Ammonia it was found that at 50 torr pressure ...
Text Solution
|
- At STP the density of a gas X is three times that of gas Y while molec...
Text Solution
|
- The ionization energy of He^(+) is x times that of H. The ionization e...
Text Solution
|
- A certain buffer solution contains equal concentartion of X^(Theta) an...
Text Solution
|
- The depression in freezing point of 0.01 m aqueous CH(3)CooH solution ...
Text Solution
|
- Consider the structure of Al(2)Me(6) compound and find the value of (x...
Text Solution
|
- Find the value of x in the tremolite asbestos : Ca(2)Mg(x)(Si(4)O(11)...
Text Solution
|
- What is the degree of unsaturation in compound (x).
Text Solution
|
- The total number of carboxylic acid groups in the product P is
Text Solution
|
- The de Broglie wavelength of a particle of mass 6 gram and velocity 10...
Text Solution
|
- In an atom with atomic no. 29 and mass no. 59,the number of electron i...
Text Solution
|