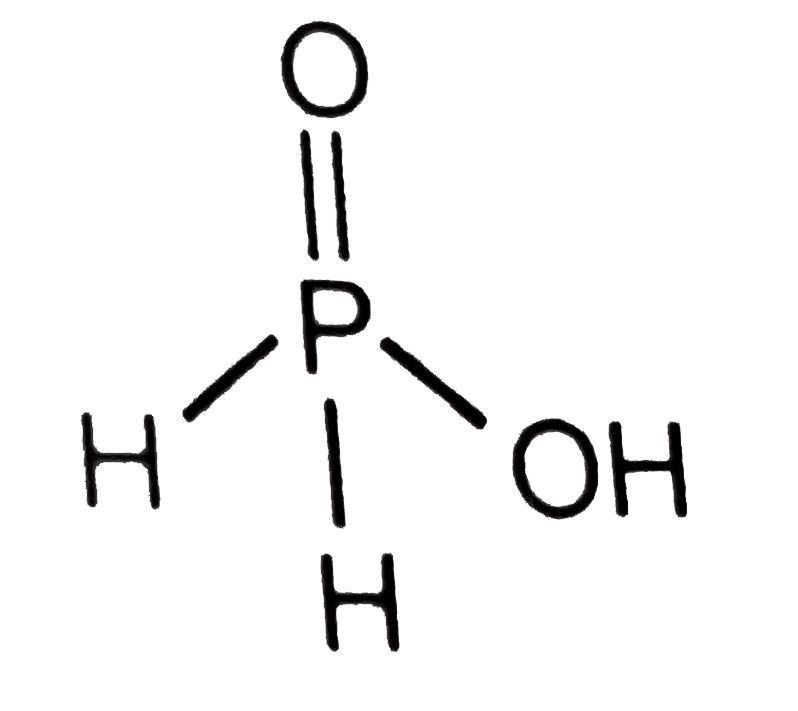
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ALP PART 1 Comprehension # 1 OBJECTIVE|8 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ALP PART 1 Comprehension # 2 OBJECTIVE|1 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ALP PART CBSC- III SUBJECTIVE|1 VideosNUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P BLOCK ELEMENTS-ALP PART 1 OBJECTIVE Assertion/ Reasonig
- Statement -1 : Hydrolysis of NCl(3) gives NH(4)OH and HOCl while PCl(...
Text Solution
|
- Statement -1 : Na(2)HPO(3) is not an acid salt. Statement -2 : Na(2)...
Text Solution
|
- Statement -1 : NO(2) and CIO(2) both being odd electron molecules dime...
Text Solution
|
- Statement -1 : H(3)PO(2) is a weak monobasic acid and is also strong r...
Text Solution
|
- assertion : Ozone is a powerful oxidising agent in comparison to O(2)....
Text Solution
|
- Statement -1 : Sodium thiosulphate is not prepared by boiling Na(2)SO(...
Text Solution
|