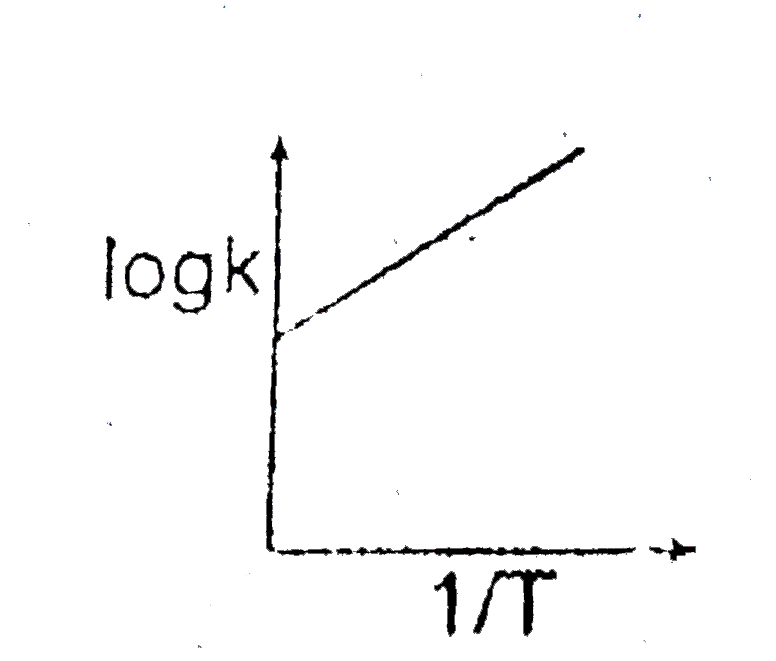
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-PT-01
- For the reactions, AhArrB, K(c)=1,BhArrC,K(c)=3,ChArrD,K(c)=5. K(c) ...
Text Solution
|
- The conversion of sugar C(12)H(22)O(11) to CO(2) is :
Text Solution
|
- If standard heat of dissociation of PCl(5) is 230 cal than slope of ...
Text Solution
|
- For a certain gas P(c) and T(c) are 80atm and -33^(@)C respectively th...
Text Solution
|
- Which of the following statement (s) is true I- Slpe of isotherm at...
Text Solution
|
- A mixture contain 61.2% BaO, 28% CaO and 10.8 % ( silica ) impurities...
Text Solution
|
- Consider the reactions (i) PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) (ii) ...
Text Solution
|
- Two glass bulbs A and B at same temperature are connected by a very sm...
Text Solution
|
- Determine enthalpy of formation for H(2)O(2)(l), using listed enthalpi...
Text Solution
|
- The rate of diffusion of two gases X and Y is in the ration of 1:5 and...
Text Solution
|
- Which one is the correct ground state electronic configuration :
Text Solution
|
- At constant temperature , the equilibrium constant (KP) for the deco...
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown in P-V diagram. T...
Text Solution
|
- In a chemical reaction, K(2)Cr(2)O(7)+xH(2)SO(4)+ySO(2) to K(2)SO(4)+ ...
Text Solution
|
- For the gas phase reaction, PCI (5)(g)Leftrightarrow OCI(3)(g) + CI(...
Text Solution
|
- The enthalpy of neutralization of a week monoprotic acid (HA) in 1 M s...
Text Solution
|
- Benzene burns according to the following equation at 300K (R=8.314J mo...
Text Solution
|
- X and Y are two elements which form X(2)Y(3) and X(3)Y(4). If 0.20 mol...
Text Solution
|
- In the reaction 4A + 2B + 3C to A(4)B(2) C(3), what will be the numb...
Text Solution
|
- The correct figure representing isothermal and adiabatic expansions of...
Text Solution
|