A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-NITROGEN CONTAINING COMPOUNDS-ORGANIC CHEMISTRY(Nitrogen containing Compounds)
- The uncertainties in position and the velocity of a particle are 10^(-...
Text Solution
|
- In the diazotisation of anline with sodium nitrite and hydrochloride a...
Text Solution
|
- Starting from propanoic acid, the following reaction were carried acid...
Text Solution
|
- In the reaction, C(6)H(5)NH(2)underset(0-5^(@)C)overset(NaNO(2)+HCl)...
Text Solution
|
- In the given reaction product P is :
Text Solution
|
- P ( major product ), P is
Text Solution
|
- Identify A,C(6)H(11)N, for which the given information is availabel . ...
Text Solution
|
- An optically inactive amine (A) is methylated with excess of CH(3)I an...
Text Solution
|
- The product P formed in the given reaction sequence is .
Text Solution
|
- Identify the correct statement.
Text Solution
|
- Which statement is incorrect.
Text Solution
|
- What will be the major product when 2-Aminopropane is treated with nit...
Text Solution
|
- Which of the following product (s) will be obtained when isopropylamin...
Text Solution
|
- Which of the following compounds will give N(2)(g) on treatment with H...
Text Solution
|
- Compound (x)(m.f=C(7)H(8)N), on reaction with NaNO(2) and conc. HCl at...
Text Solution
|
- Pyridine is less basic than triethylamine because
Text Solution
|
- The reagents are
Text Solution
|
- Arrange following Amines for rate of reaction with CHCl(3)+KOH ?
Text Solution
|
- Which of the following is correct order of basic strength for the give...
Text Solution
|
- Which nitrogen atom is most basic ?
Text Solution
|


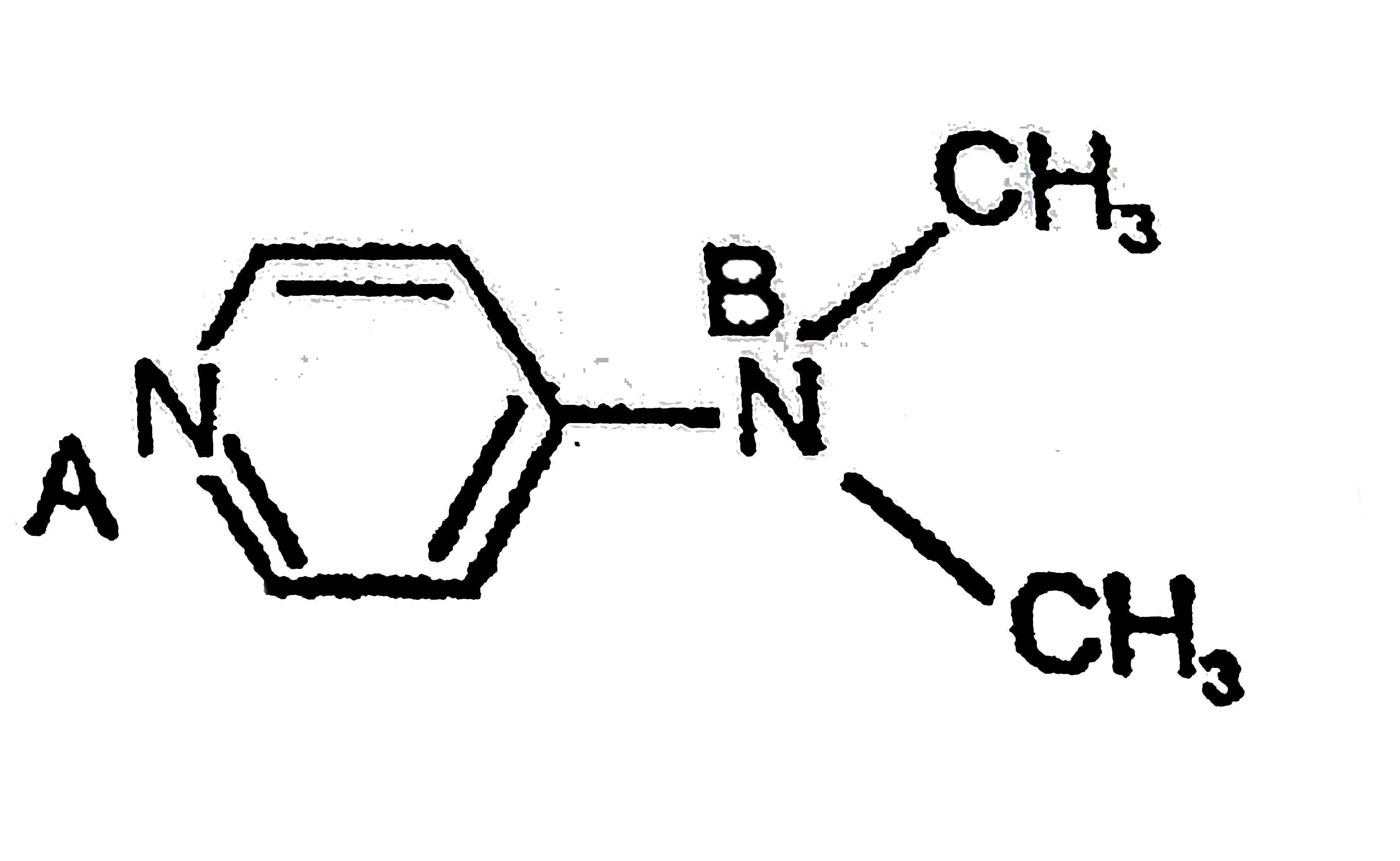 A is more basic than B
A is more basic than B