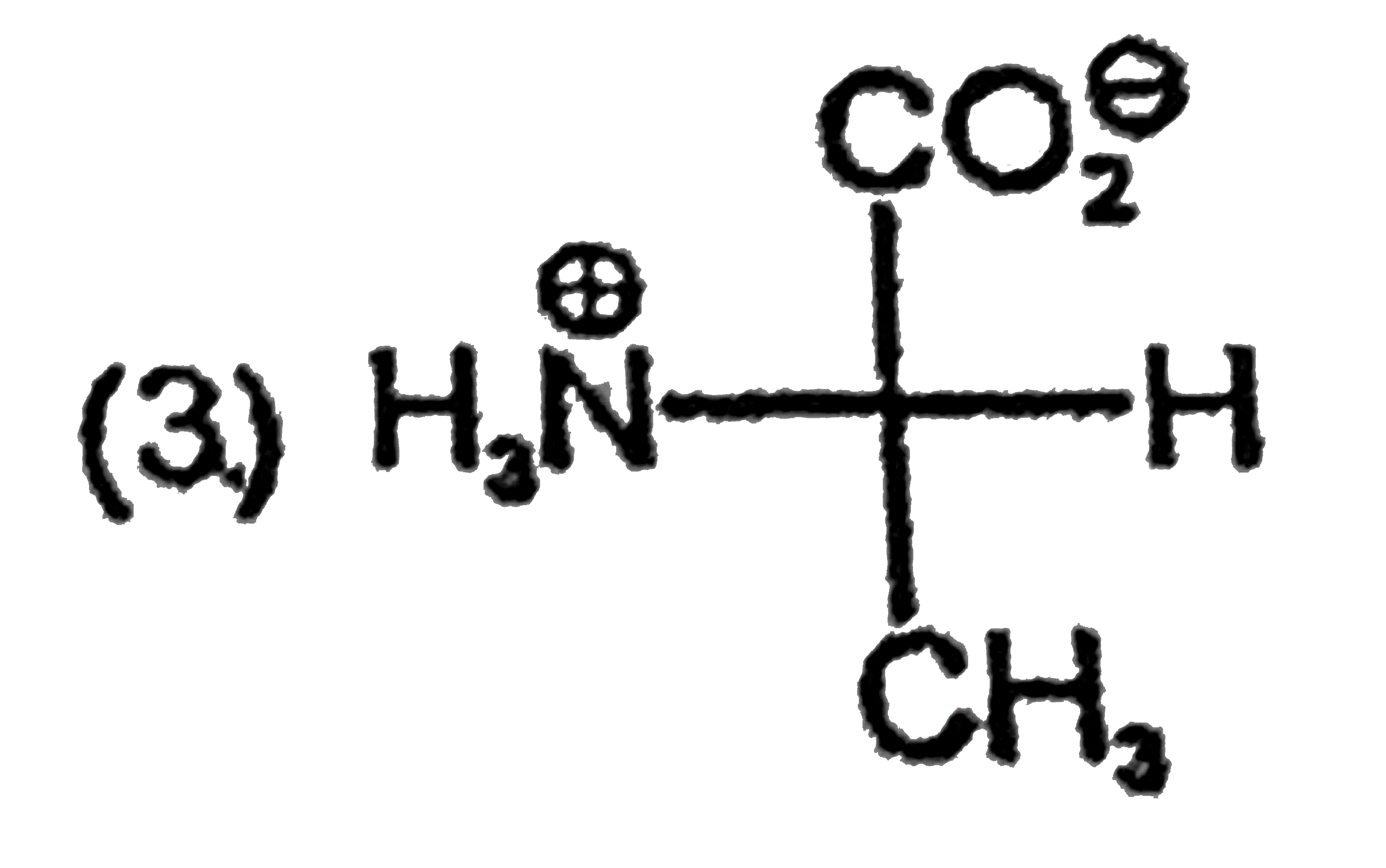
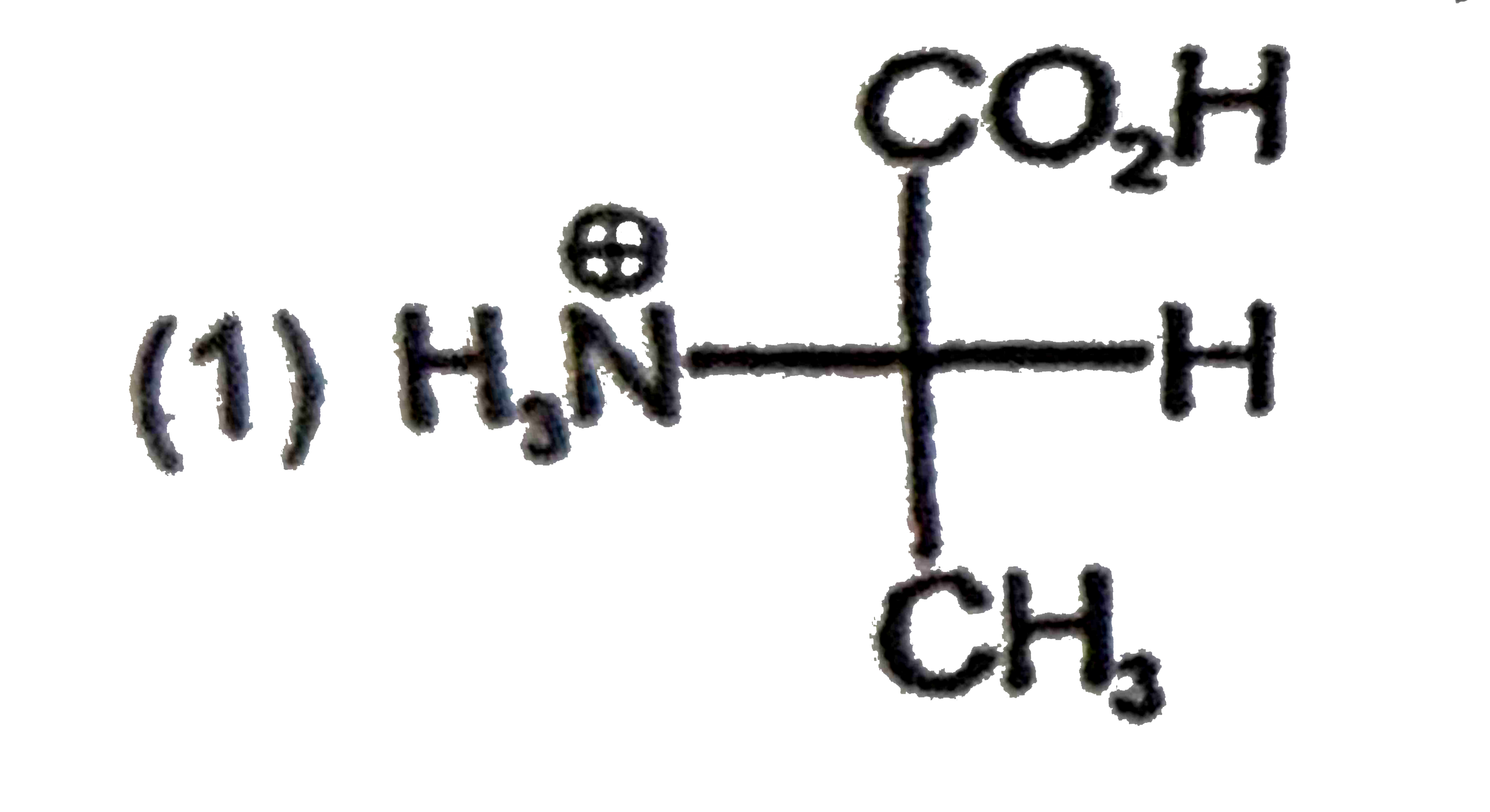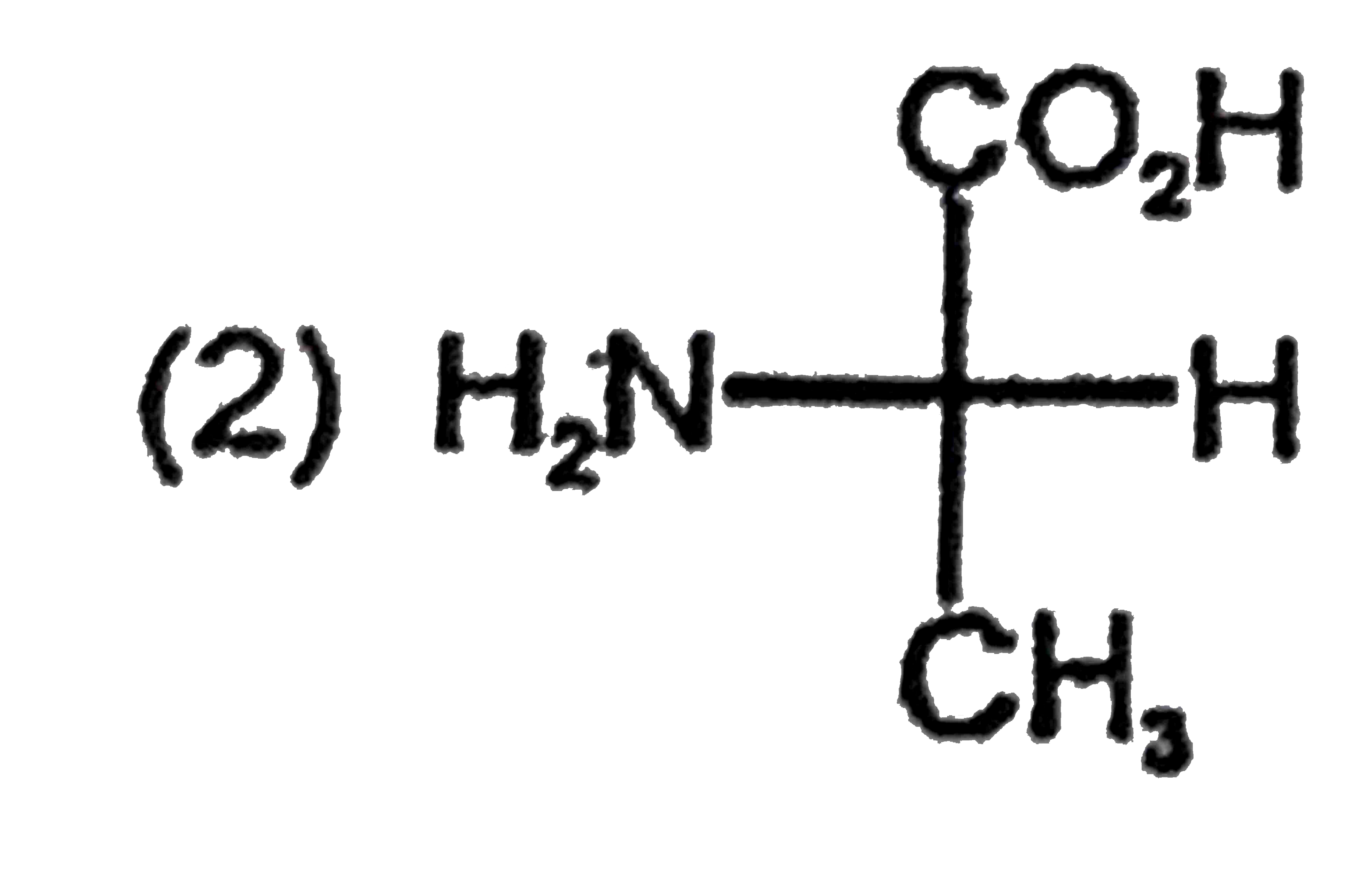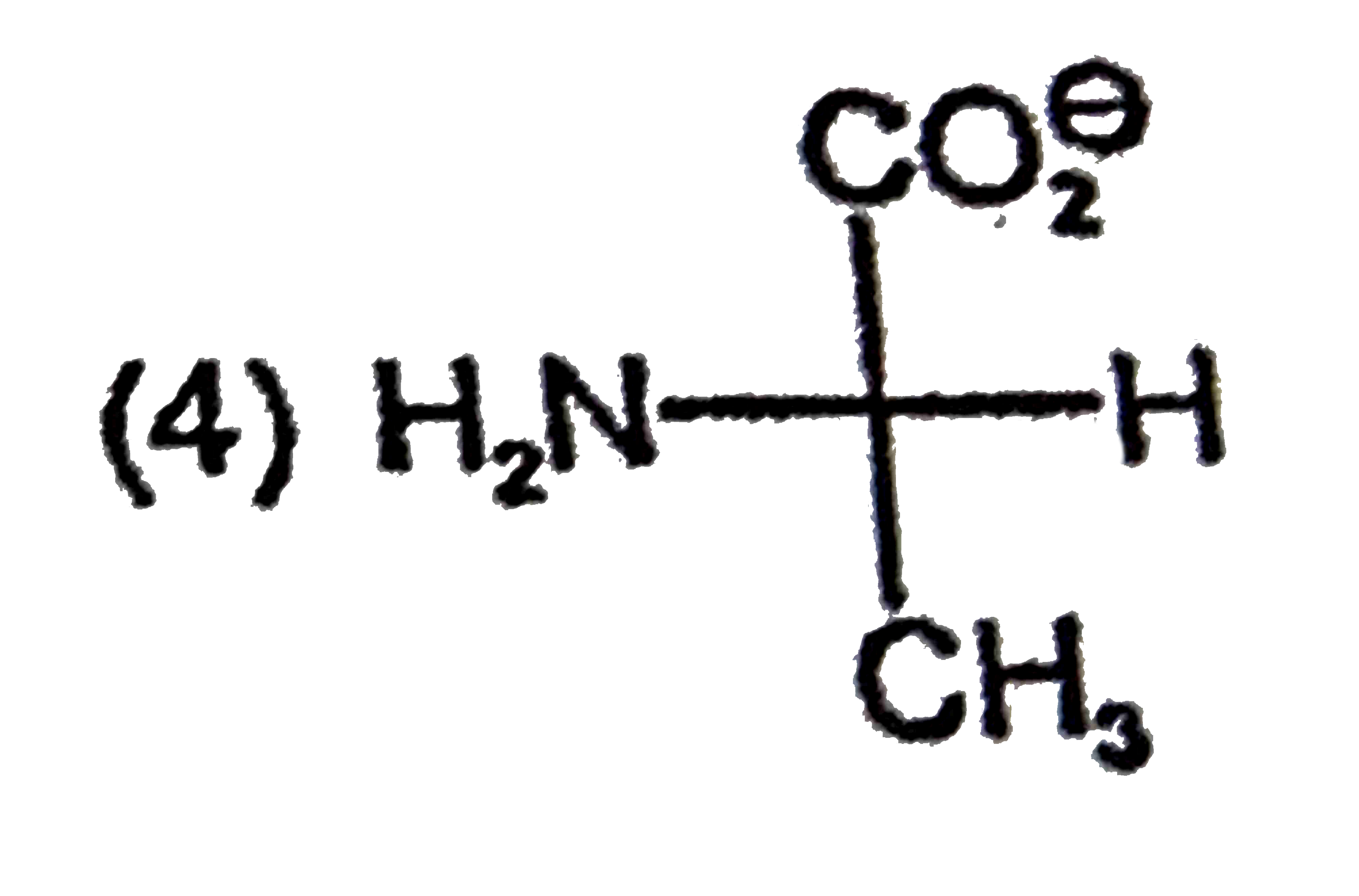A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-BIOMOLECULES & POLYMER-ORGANIC CHEMISTRY(Biomolecules & Polymer)
- Which two of the following aldohexoses give the same osazone derivativ...
Text Solution
|
- A hexapeptide has the composition Ala,Gly,Phe,Val. Both the N- termina...
Text Solution
|
- Which of the following is the major solute species in a solution of al...
Text Solution
|
- Glucose is
Text Solution
|
- The above process in which alpha and beta form remain in equilibrium w...
Text Solution
|
- Glucose on reduction with Na//Hg and water gives?
Text Solution
|
- Which of the following pair give positive Tollen's Test ?
Text Solution
|
- Product P & Q may be grouped as
Text Solution
|
- How many moles of acetic anhydride (Ac(2)O) is needed to react comple...
Text Solution
|
- Glucose reacts with bromine water to products :
Text Solution
|
- Which one of the following statements is not true regarding (+) lactos...
Text Solution
|
- The following carbohydrate is:
Text Solution
|
- Test by which starch and cellulose can be distinguished from each othe...
Text Solution
|
- Which of the following disaccharides will not reduce tollen's reagent.
Text Solution
|
- (+)Arabinose is (2R, 3S,4S)-aldopentose which of the following is (+)-...
Text Solution
|
- The osmotic pressure of blood is 5.65 atm at 27^(@) C.The number of mo...
Text Solution
|
- Which of the statement is incorrect.
Text Solution
|
- Which of the following is not reducing sugar.
Text Solution
|
- underset(" "CH(2)OH)underset(|)underset(" "(CHOH)(3))underset...
Text Solution
|
- The incorrect structure of glycine at given pH are :
Text Solution
|