A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-BIOMOLECULES & POLYMER-ORGANIC CHEMISTRY(Biomolecules & Polymer)
- (+)Arabinose is (2R, 3S,4S)-aldopentose which of the following is (+)-...
Text Solution
|
- The osmotic pressure of blood is 5.65 atm at 27^(@) C.The number of mo...
Text Solution
|
- Which of the statement is incorrect.
Text Solution
|
- Which of the following is not reducing sugar.
Text Solution
|
- underset(" "CH(2)OH)underset(|)underset(" "(CHOH)(3))underset...
Text Solution
|
- The incorrect structure of glycine at given pH are :
Text Solution
|
- Threonine is (2S,3R) -2-amino-3-hydroxybutanoic acid. Which of the fol...
Text Solution
|
- Two aldopentoses A and B given the same osazone derivative A is oxidiz...
Text Solution
|
- Which of the following is not an important secondary structural featur...
Text Solution
|
- Which of the following is the major solute species in a solution of ly...
Text Solution
|
- The commercial name of polymethyl methacrylate (P M M A) is :
Text Solution
|
- Which of the structure represent methyl alpha -D- galactopyranoside ?
Text Solution
|
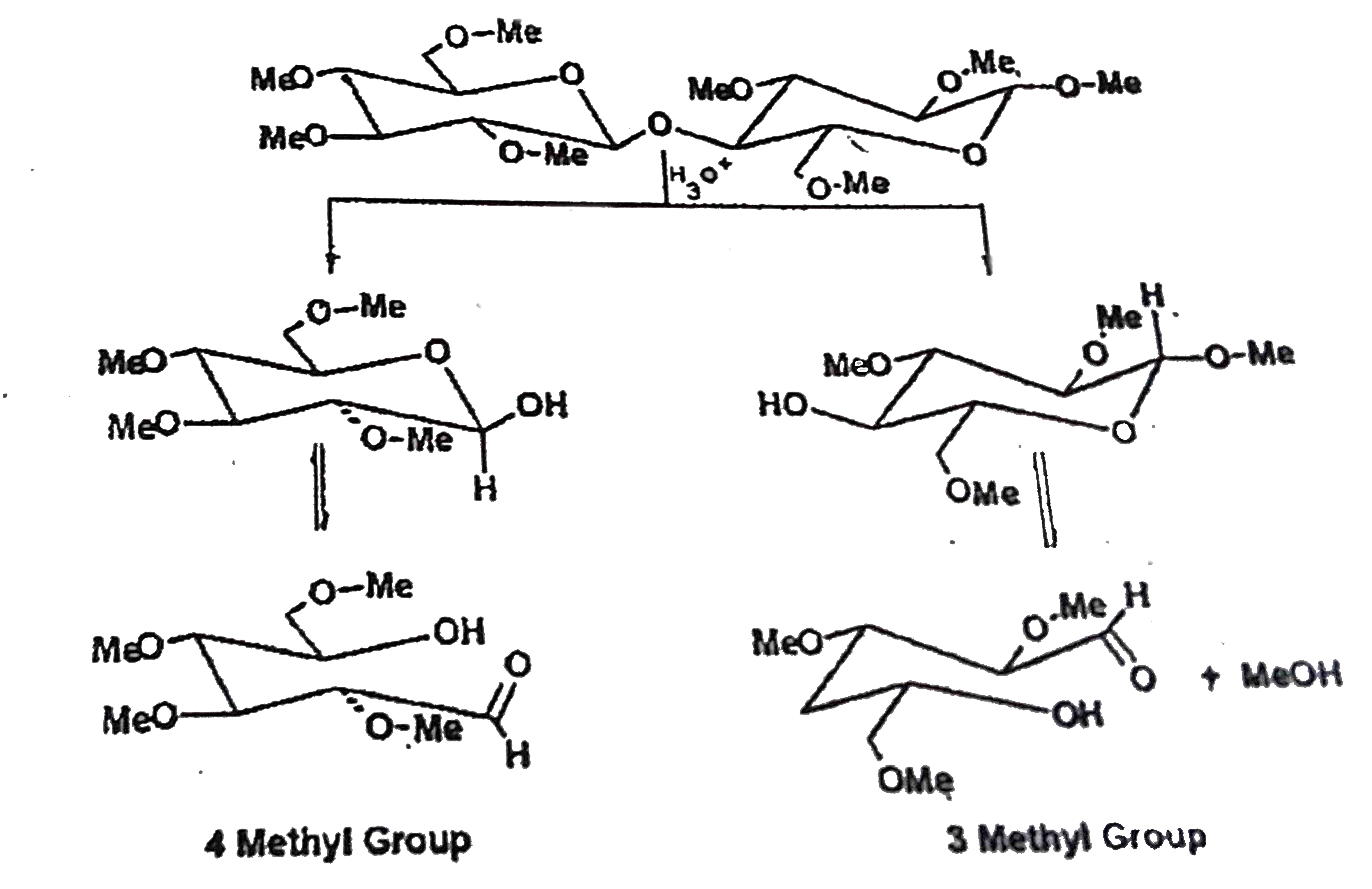
- When octa -O- methyl D- cellobiose is hydrolyzed by aqueous acid, two ...
Text Solution
|
- Which of the following is vitamin A ?
Text Solution
|
- What is the complementary m-RNA sequence for the DNA segment AATCAGTT?
Text Solution
|
- Which of the following gives an optically inactive aldaric acid on oxi...
Text Solution
|
- Which two of the following compounds, if any, are epimers ?
Text Solution
|
- Which statement is incorrect regarding alkali metals.
Text Solution
|
- An amino acid is characterized by two pKa values the one corresponding...
Text Solution
|
- Which of the following statements is wrong for the solution of alkali ...
Text Solution
|