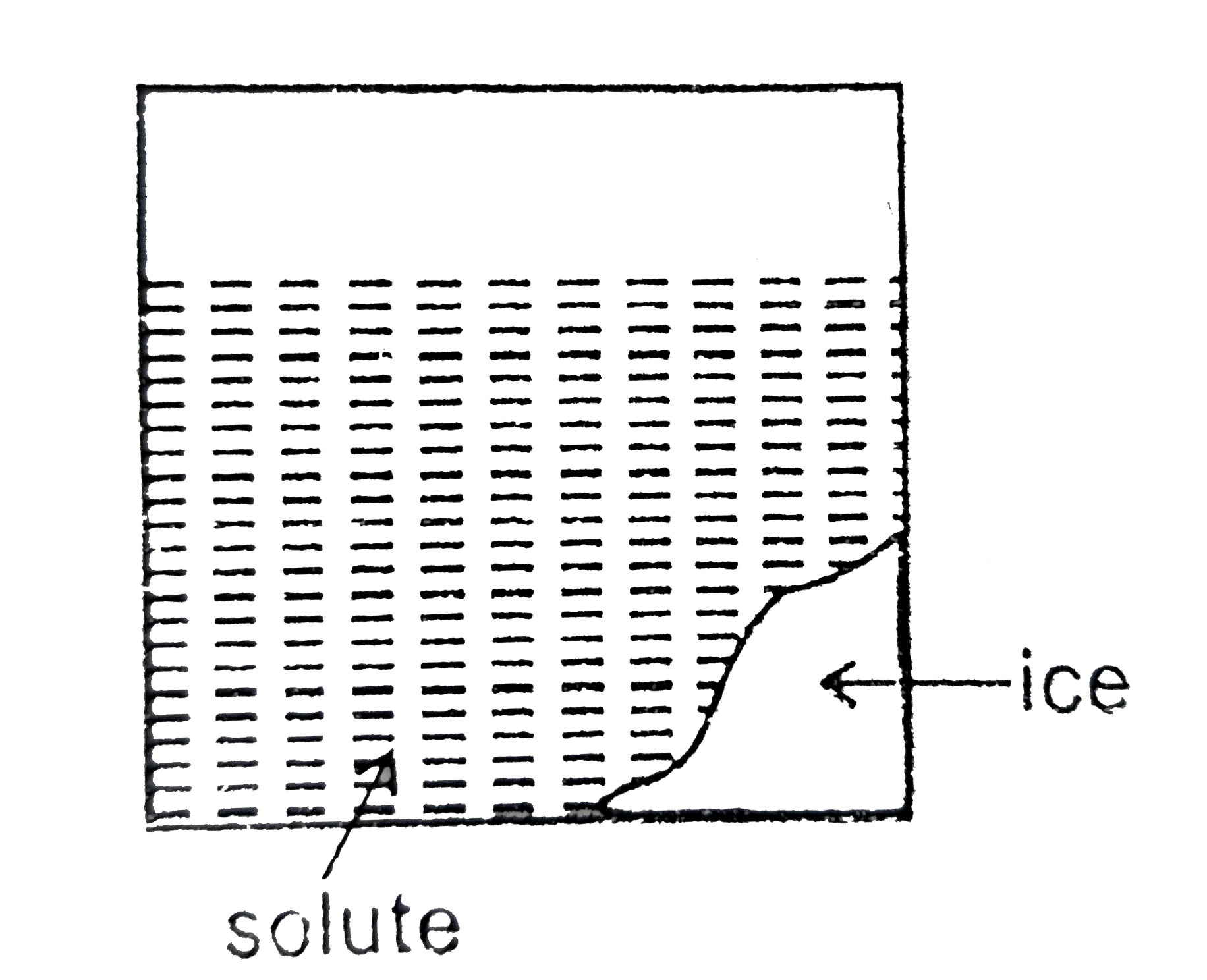
Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
RESONANCE ENGLISH|Exercise (MSPs)|7 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise Board Level Exercise|28 VideosSOLUTION AND COLLIGATIVE PROPERTIES
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)|52 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|23 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-SOLUTIONS-Advabced Level Problems (PART-2)
- 1000gm H(2)O have 0.1 mole urea and its freezing point is-0.2^(@)C and...
Text Solution
|
- The osmotic pressure of 6% solution of urea at 300 K is
Text Solution
|
- The osmotic pressure of 6% solution of urea at 273 K is
Text Solution
|
- The osmotic pressure of 6% solution of urea at 323 K is
Text Solution
|
- There are two solutions each at 27^(@)C Solution A: contains 6g urea...
Text Solution
|
- The osmotic pressure of 6% solution of urea at 298 K is
Text Solution
|
- The osmotic pressure of 6% solution of urea at 258 K is
Text Solution
|
- The osmotic pressure of 5% solution of urea at 258 K is
Text Solution
|
- The osmotic pressure of 7% solution of urea at 298 K is
Text Solution
|
- The osmotic pressure of 8% solution of urea at 300 K is
Text Solution
|
- The freezing point depression of 0.001 m K(x) [Fe(CN)(6)] is 7.10xx10^...
Text Solution
|
- Pure water can be obtained from sea water by:
Text Solution
|
- The osmotic pressure of a solution containing 0.3 mol of solute per li...
Text Solution
|
- The osmotic pressure of a solution containing 0.4 mol of solute per li...
Text Solution
|
- An aqueous solution containing 288gm of a non-volatile compound havin...
Text Solution
|
- The osmotic pressure of a solution containing 0.3 mol of solute per li...
Text Solution
|
- The osmotic pressure of a solution containing 0.2 mol of solute per li...
Text Solution
|
- Calculate the boiling point of water at 700mm pressure of Hg. The heat...
Text Solution
|
- The osmotic pressure of a solution containing 0.1 mol of solute per li...
Text Solution
|
- An aqueous solution of glucose boils at 100.01^(@)C.The molal elevatio...
Text Solution
|
- The osmotic pressure of a solution containing 0.2 mol of solute per li...
Text Solution
|