A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
RESONANCE ENGLISH|Exercise Advabced Level Problems (PART-2)|35 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise EXERCISE-3(PART-3)|30 VideosSOLUTION AND COLLIGATIVE PROPERTIES
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)|52 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|23 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-SOLUTIONS-Advabced Level Problems (PART-1)
- If relative decrease in vapour pressure is 0.4 for a solution containi...
Text Solution
|
- Which of the following azeotropic solution has the b.p. less than b.p...
Text Solution
|
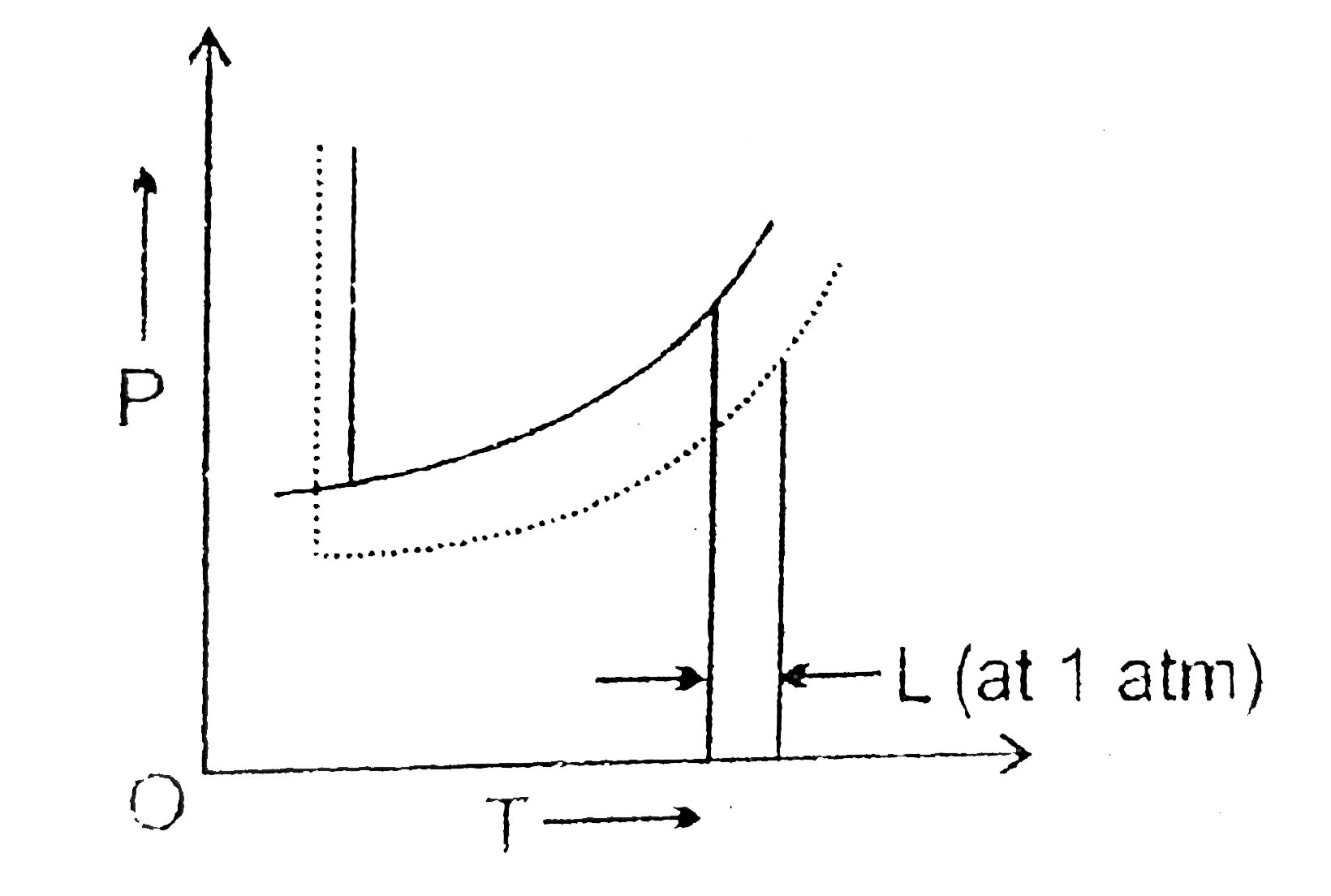
- The phase diagrams for the pure solvent (solid lines) and the solution...
Text Solution
|
- The total concentration of dissolved particles in side red blood cells...
Text Solution
|
- The fundamental cause of DeltaT (depression is):
Text Solution
|
- Vapour pressure of C CL(4) at 25^@C is 143 mmHg 0.05g of a non-volatil...
Text Solution
|
- A 0.50 molal solution of ethylene glycol in water is used as coolant i...
Text Solution
|
- The depression of freezing points of 0.05 molal aqueous solution of th...
Text Solution
|
- Assuming each salt to be 90% dissociated which of the following will h...
Text Solution
|
- The boiling point of an azeotropic mixture of water and ethyl alcohol ...
Text Solution
|
- On mixing 10 mL of acetone with 40mL of chloroform, the total volume o...
Text Solution
|
- A teacher one day pointed out to his students the peculiar fact that w...
Text Solution
|
- When an ideal binary solution is in equilibrium with its vapour, molar...
Text Solution
|
- The melting points of most of the solid substances increases with an i...
Text Solution
|
- For an ideal binary liquid solutions with P(A)^(@)gtP(B)^(@), which re...
Text Solution
|
- What will be the molecular weight of NaCl determined experimentally fo...
Text Solution
|
- Which characterises the weak intermolecular forces of attraction in a ...
Text Solution
|
- A liquid is in equilibrium with its vapour at its boiling point. On th...
Text Solution
|
- On the basic intermolecular force predict the correct order of decre...
Text Solution
|
- During depression of freezing point in a solution, the following are i...
Text Solution
|