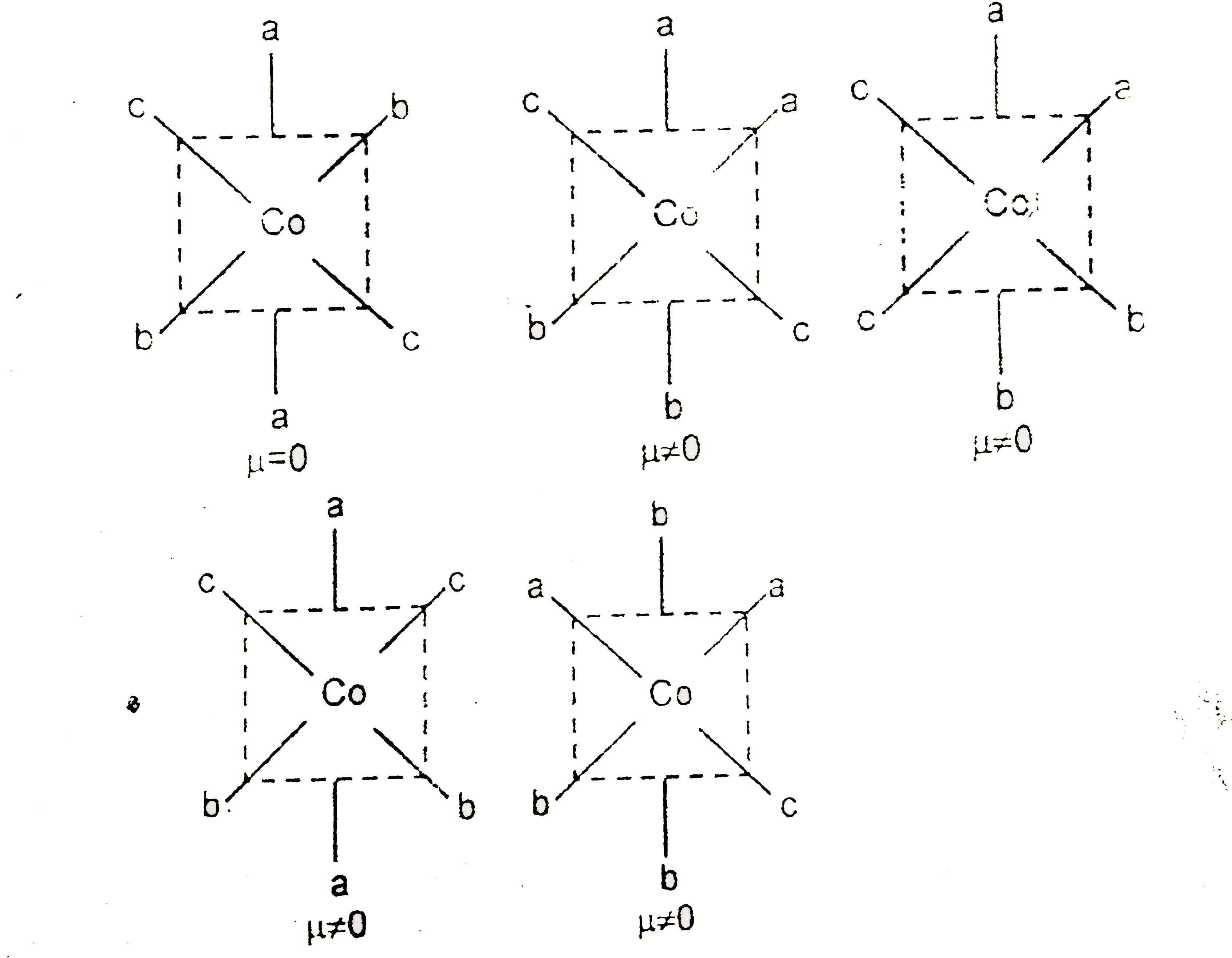
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- State whether the given statement is correct or not : An azeotropic ...
Text Solution
|
- The solution which boil at constant temperature like a pure liquid and...
Text Solution
|
- An azeotropic solution of two liquid has boiling point lower than eith...
Text Solution
|
- Two liquids are mixed together to from a mixture which boils at same t...
Text Solution
|
- An azeotropic solution of two liquids has a boiling point higher than ...
Text Solution
|
- An azeotropic solution of two liquids has a boiling point lower than e...
Text Solution
|
- An azeotropic mixture of two liquids has boiling point lower than eith...
Text Solution
|
- दो द्रवों का स्थिर क्वथनांकी (azeotropic) मिश्रण दोनों शुद्ध द्रवों के...
Text Solution
|
- An azeotropic solution of two liquids has a boiling point lower than e...
Text Solution
|