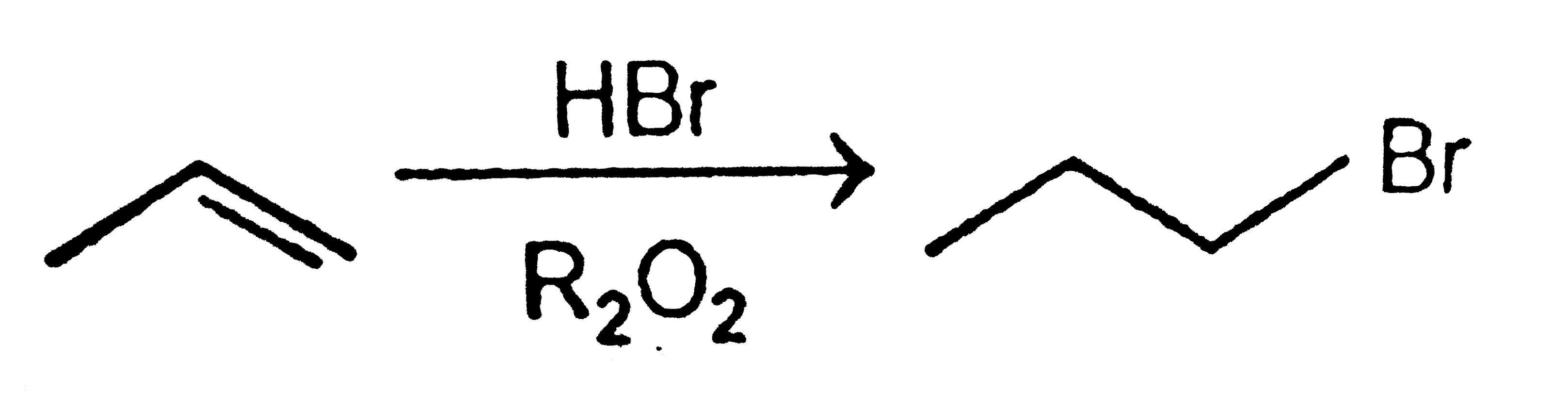
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the number of intermediate which is involved in the given reacti...
Text Solution
|
- An organic reaction occurs by using reagents called electrophiles and ...
Text Solution
|
- An organic reaction occurs by using reagents called electrophiles and ...
Text Solution
|
- Write the number of intermediate which is involved in the given reacti...
Text Solution
|
- Which of the following reactions involve free radical as intermediate ...
Text Solution
|
- Classify each of the following as homolysis as homolysis or heterolysi...
Text Solution
|
- The most Carbocations, carbanions, free radicals and radical cation ar...
Text Solution
|
- What is free radicals, carbanions and carbocations? How they form?
Text Solution
|
- What is free radicals, carbanions and carbocations? How they form?
Text Solution
|