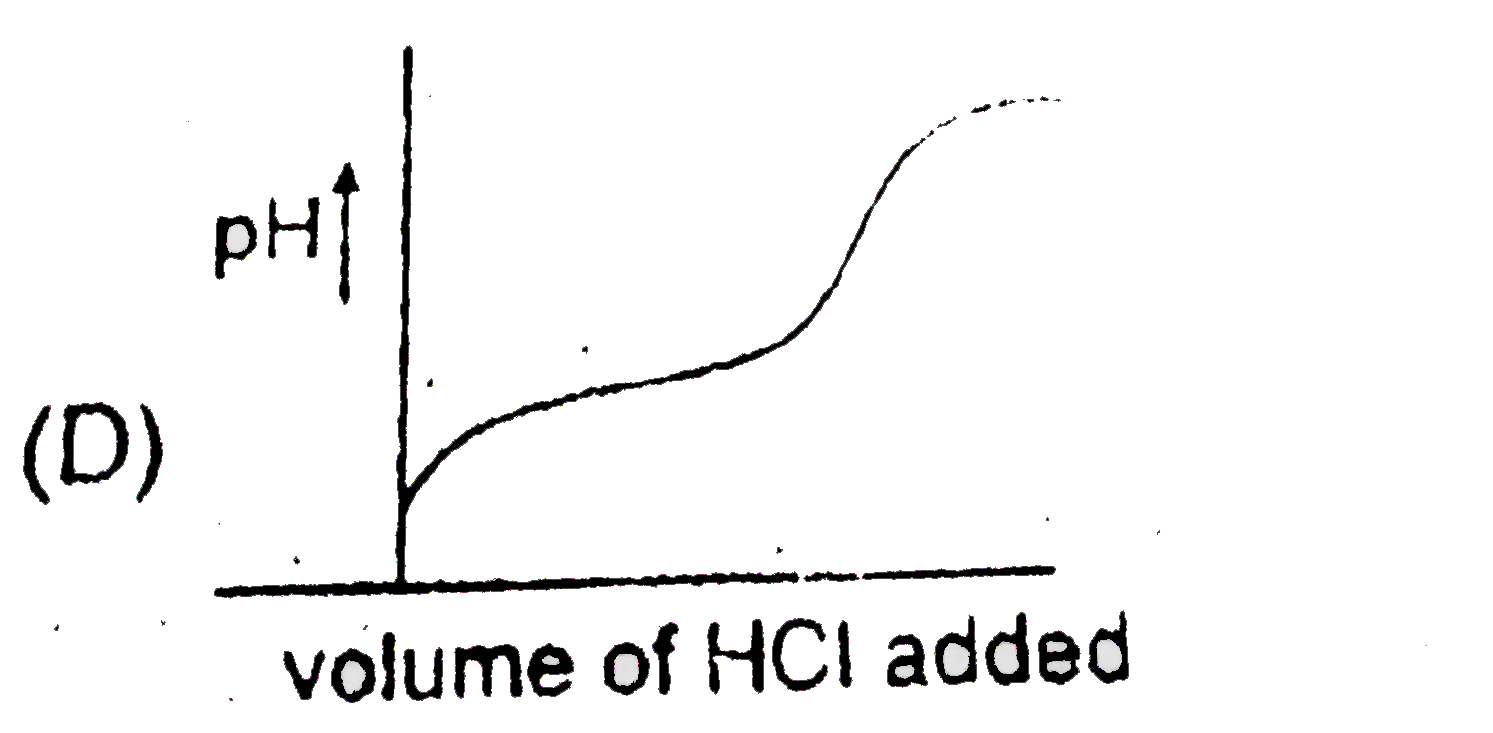
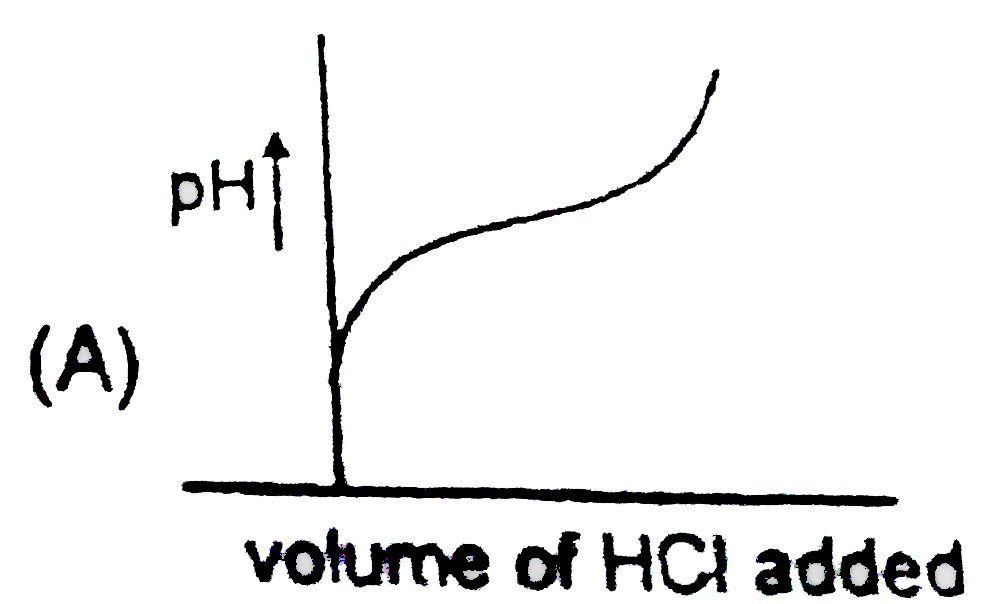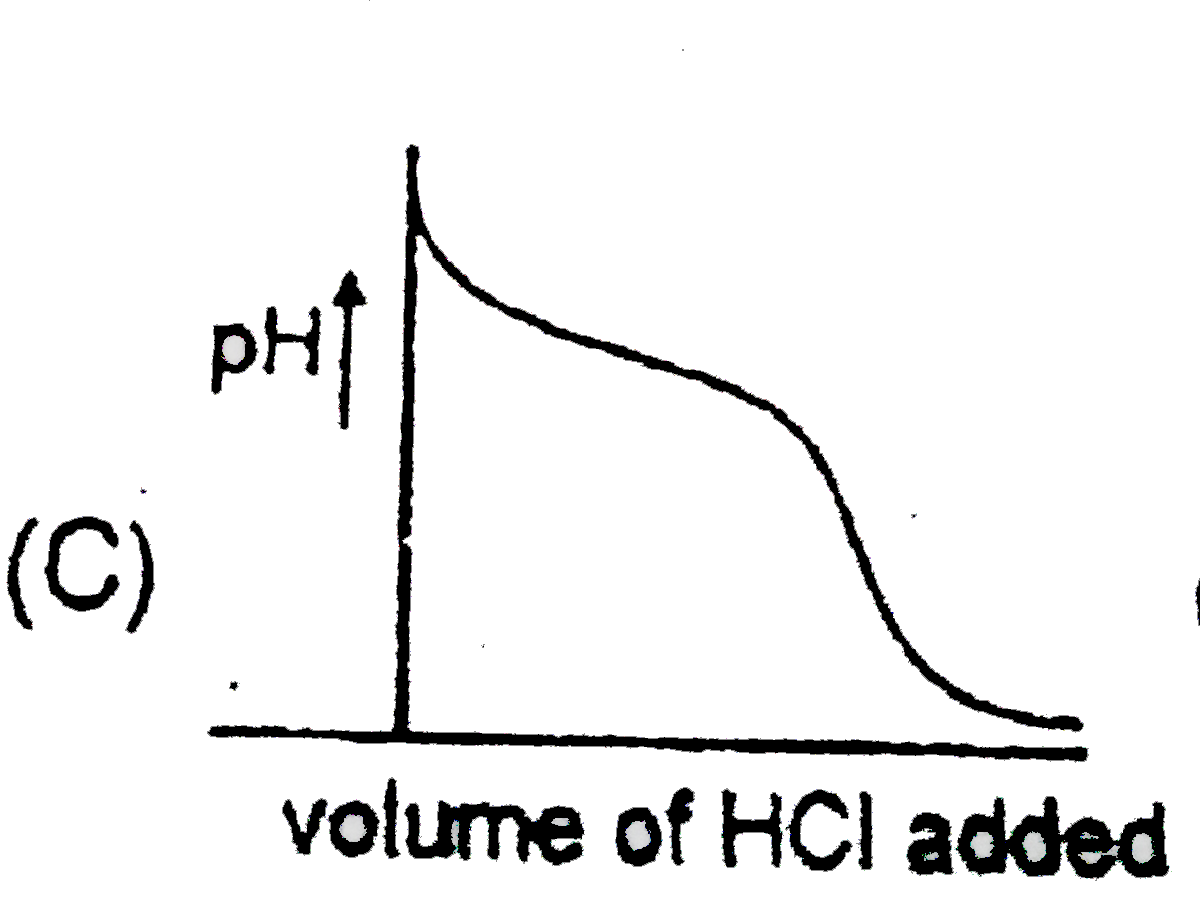A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Find DeltapH when 100 ml of 0.01 M HCl is added in a solution containi...
Text Solution
|
- The ionization contant of benzoic acid is 6.46xx10^(-5) and K(sp) for ...
Text Solution
|
- When 100 mL of 0.1 M NaCN solution is titrated with 0.1 M HCl solution...
Text Solution
|
- The indicator constant for an acidic indicator, HIn is 5xx10^(-6)M. Th...
Text Solution
|
- Ionisation constant of HA (weak acid) and BOH (weak base) are 3.0xx10^...
Text Solution
|
- Which of the following concentration of NH(4)^(+) will be sufficient t...
Text Solution
|
- pOH=7-0.5pKa+0.5 pKb is true for aqueous solution containing which pai...
Text Solution
|
- An acid-base indicator which is a weak acid has a pK(a) value=5.45. At...
Text Solution
|
- Find the no. of moles of O2 present in 2.01x10^23 molecules of oxygen ...
Text Solution
|
- A certain mixture of HCl and CH3-COOH is 0.1 M in each of the acids.20...
Text Solution
|
- A buffer solution is made by mixing weak acid HA (K(a)=10^(-6)) with i...
Text Solution
|
- A sample of water has a hardness expressed as 80 ppm of Ca^(2+) This s...
Text Solution
|
- To a 100 mL of 0.1 M weak acid HA solution 22.5 mL of 0.2 M solution o...
Text Solution
|
- When 100 mL of 0.1 M NaCN solution is titrated with 0.1 M HCl solution...
Text Solution
|
- The variation of pH during the titration of 0.5 N Na2CO3 with 0.5 N HC...
Text Solution
|
- Which of the following solutions when added to 1L of a 0.1 M CH3COOH w...
Text Solution
|
- A 1 litre solution of pH=1 diluted upto 10 times.What volume of a solu...
Text Solution
|
- Select the correct statements:
Text Solution
|
- A mixture of NO2 and N2O4 has a vapour density of 38.3 at 300K. What i...
Text Solution
|
- Statement-1:Phenolphathalein can be used as indicator in the titration...
Text Solution
|