Consider the following system.
Three different aqueous solution each having volume 100 ml are taken are kept in contact as shown.
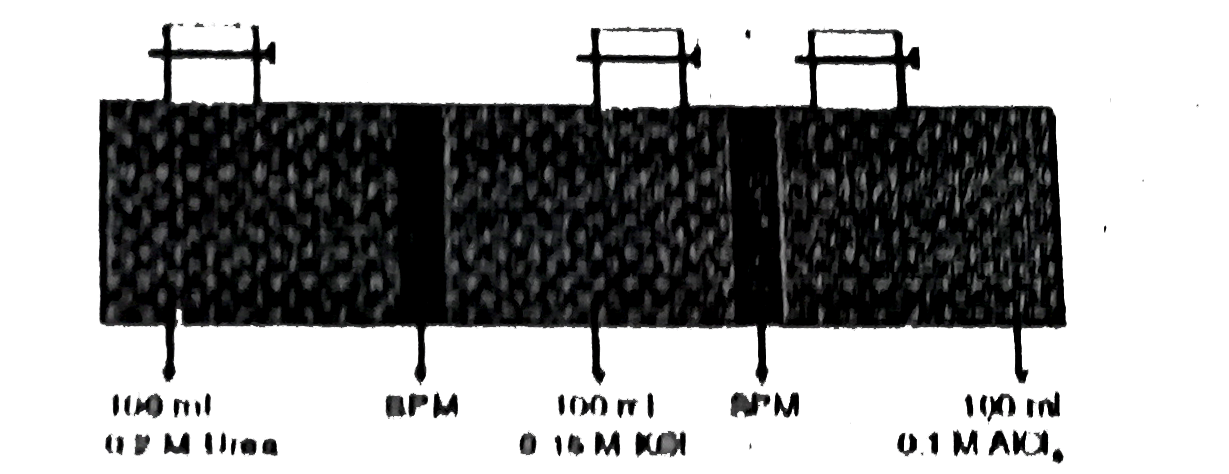
After sufficient time (Consider temp constant & 100% dissociation of strong electrolyte )
Consider the following system.
Three different aqueous solution each having volume 100 ml are taken are kept in contact as shown.

After sufficient time (Consider temp constant & 100% dissociation of strong electrolyte )
Three different aqueous solution each having volume 100 ml are taken are kept in contact as shown.

After sufficient time (Consider temp constant & 100% dissociation of strong electrolyte )
A
Volume of urea solution will be `100/3` ml.
B
Volume of `AlCl_3` solution will be `400/3` ml.
C
There will be no change in volume of KCl solution
D
Volume of both KCl and `AlCl_2` solutions will increase.
Text Solution
Verified by Experts
The correct Answer is:
BC
After sufficient time osmotic pressure of all solution will become same.
as T is same i.e. molar concentration should be same, for this ratio of volume should be the same as that of ratio moles.
`{:("Urea",:,KCl,:,AlCl_3),(20 m "moles", " " ,30 m "moles", " " ,40 m "moles"):}`
Total volume (300 ml) should be divided in 2:3:4
`V_("urea")=2/9xx300=200/3 ml`
`V_(KCl)=3/9xx300=100 ml`
`V_(AlCl_3)=4/9xx300=400/3`ml
as T is same i.e. molar concentration should be same, for this ratio of volume should be the same as that of ratio moles.
`{:("Urea",:,KCl,:,AlCl_3),(20 m "moles", " " ,30 m "moles", " " ,40 m "moles"):}`
Total volume (300 ml) should be divided in 2:3:4
`V_("urea")=2/9xx300=200/3 ml`
`V_(KCl)=3/9xx300=100 ml`
`V_(AlCl_3)=4/9xx300=400/3`ml
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
An ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding P-V diagrams in column 3 of the table. Consider only the path from state 1 to state 2. W denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic process. Here is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas in n. What one of the following options correctly represents a thermodynamic process that is used as a correction in the determination of the speed of sound in an ideas gas?
An ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding P-V diagrams in column 3 of the table. Consider only the path from state 1 to state 2. W denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic process. Here is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas in n. Which of the following options is the only correct representation of a process in which Delta U = Delta Q - P Delta V ?
Two blocks of masses m_(1) and m_(2) are connected with a light spring of force constant k and the whole system is kept on a frictionless horizontal surface. The masses are applied forces F_(1) and F_(2) as shown in fig. At any time the blocks have same acceleration a_(0) but in opposite direction. Now answer the following : If F_(2) is removed at this moment, then just after the acceleration of m_(2) is
The colloidal particles are electrically charged as a indicated by their migration towards cathode or anode under the applied electric field. In a particular colloidal system, all particles carry either positive charge or negative charge. The electric charge on colloidal particles orginate in several ways. According to preferential adsorption theory, the freshly obtained precipitate particles adsorb ions from the dispersion medium, which are common to their lattice and acquire the charge of adsorbed ions. For example, For example, freshly obtained Fe(OH)_(3) precipitated is dispersed, by a little FeCl_(3) , into colloidal solution owing to the adsorption of Fe^(3+) ions in preference. Thus sol particles will be positively charged. In some cases the colloidal particles are aggregates of cations or anions having ampiphilic character. When the ions posses hydrophobic part (hydrocarbon end) as well as hydrophilic part (polar end group), they undergo association in aqueous solution to form particles having colloidal size. The formation of such particles, called micelles plays a very important role in the solubilization of water insoluble substances, (hydrocarbon, oils, fats, grease etc.). In micelles, the polar end groups are directed towards water and the hydrocarbon ends into the centre. The charge on sol particles of proteins depends on the pH. At low pH, the basic group of protein molecule is ionized (protonated) and at higher pH (alkaline medium), the acidic group is ionized. At isoelectric pH, characteristic to the protein, both basix and acidic groups are equally ionized. The stability of colloidal solution is attributed largely to the electric charge of the dispersed particles. This charge causes them to be coagulated or precipitated. On addition of small amount of electrolytes, the ions carrying oppiste charge are adsorbed by sol particles resulting in the neutralization of their charge. When the sol particles either with no charge or reduced charge, come closer due to Brownian movement, they coalesce to form bigger particles resulting in their separation from the dispersion medium. This is what is called coagulating or precipitation of the colloidal solution. The coagulating power of the effective ion, which depend on its charge, is expressed in terms of its coagulating value, defined as its minimum concentration (m mol/L) needed to precipitate a given sol. 100 ml each of two sols of AgI, one obtained by adding AgNO_(3) to slight excess of KI and another obtained by adding KI to slight excess of AgNO_(3) , are mixed together. Then :
A cyclotron is device for accelerating ions and charged particles. It was developed by Lawerence in 1932. The heart of the appritus consists of a split metal pillbox. Figure sHOws top and front views of the halves called dees. A rapidly oscilating potential difference is applied between the Dees. This produces an oscilating electric field in the gap between the dees, the region inside each dee being essentially free of electric field. The Dees are enclosed in an evaccutaed container, and the entire unit is placed in a uniform magnetic field B whose direction is normal to the plane Of Dees, A charged particle of mass 'm' and charge 'q' in the gap between the dees, it moves with constant speed in a semi-circle. The period of uniform circular motion is T=(2pim)/(qB) and is independant of speed. If the time-period of the oscilating elctric field is equal to this time, then the charged particle will be accelerated again and again Answer the following questions (consider the mass of particles remains constant during motion): A cyclotron has been adjusted to accelereted deutrons. It is now to be adjusted to accelerate other particles , for this following changes may be made: (a) In order to keep the frequency of oscillating electric field same, the magnetic field is halved for proton (b) If the magnetic field remain unchanged, the oscillation frequency of electric field should be halved for alpha particle (c ) If the magnetic field remain unchanged, the oscillation frequency of electric field should be double for proton (d) If the frequency of oscillating electric field is kept same, the magnetic field should be kept same for alpha particle
The word fluid means a substance having particles which readily of its magnitude (a small shear stress, which may appear to be of negligible will cause deformation in the fluid). Fluids are charactrised by such properties as density. Specific weight, specific gravity, viscosity etc. Density of a substance is defined as mass per unit volume and it is denoted by. The specific gravity represents a numerical ratio of two densities, and water is commonly taken as a reference substance. Specific gravity of a substance in written as the ratio of density of substance to the density of water. Specific weight represents the force exerted by gravity on a unit volume of fluid. It is related to the density as the product of density of a fluid and acceleration due to gravity. Viscosity is the most important and is recognized as the only single property which influences the fluid motion to a great extent. The viscosity is the property by virtue of which a fluid offers resistance to deformation under the influenece if shear force. The force between the layers opposing relative motion between them are known as forces of viscosity. When a boat moves slowly on the river remains at rest. Velocities of different layers are different. Let v be the velocity of the level at a distance y from the bed and V+dv be the velocity at a distance y+dy . The velocity differs by dv in going through a distance by perpendicular to it. The quantity (dv)/(dy) is called velocity gradient. The force of viscosity between two layers of a fluid is proportional to velocity gradient and Area of the layer. F prop A & F prop (dv)/(dy) F= -etaA(dv)/(dy) ( -ve sign shown the force is frictional in nature and opposes relative motion. eta coefficient of dynamic viscosity Shear stress (F)/(A)= -eta(dv)/(dy) and simultaneously kinematic viscosity is defined as the dynamic viscosity divided by the density. If is denoted as v . The viscosity of a fluid depends upon its intermolecular structure. In gases, the molecules are widely spaced resulting in a negligible intermolecular cohesion, while in liquids the molecules being very close to each other, the cohesion is much larger with the increases of temperature, the cohesive force decreases rapidly resulting in the decreases of viscosity. In case of gases, the viscosity is mainly due to transfer of molecular momentum in the transerve direction brought about by the molecular agitation. Molecular agitation increases with rise in temperature. Thus we conclude that viscosity of a fluid may thus be considered to be composed of two parts, first due to intermolecuar cohesion and second due to transfer of molecular momentum. If the velocity profile is given by v=(2)/(3)y-y^(2)v is velocity in m//sec y is in meter above the bad. Determine shear stress at y=0.15m , & eta=0.863 Ns//m^(2)
The word fluid means a substance having particles which readily of its magnitude (a small shear stress, which may appear to be of negligible will cause deformation in the fluid). Fluids are charactrised by such properties as density. Specific weight, specific gravity, viscosity etc. Density of a substance is defined as mass per unit volume and it is denoted by. The specific gravity represents a numerical ratio of two densities, and water is commonly taken as a reference substance. Specific gravity of a substance in written as the ratio of density of substance to the density of water. Specific weight represents the force exerted by gravity on a unit volume of fluid. It is related to the density as the product of density of a fluid and acceleration due to gravity. Viscosity is the most important and is recognized as the only single property which influences the fluid motion to a great extent. The viscosity is the property by virtue of which a fluid offers resistance to deformation under the influenece if shear force. The force between the layers opposing relative motion between them are known as forces of viscosity. When a boat moves slowly on the river remains at rest. Velocities of different layers are different. Let v be the velocity of the level at a distance y from the bed and V+dv be the velocity at a distance y+dy . The velocity differs by dv in going through a distance by perpendicular to it. The quantity (dv)/(dy) is called velocity gradient. The force of viscosity between two layers of a fluid is proportional to velocity gradient and Area of the layer. F prop A & F prop (dv)/(dy) F= -etaA(dv)/(dy) ( -ve sign shown the force is frictional in nature and opposes relative motion. eta coefficient of dynamic viscosity Shear stress (F)/(A)= -eta(dv)/(dy) and simultaneously kinematic viscosity is defined as the dynamic viscosity divided by the density. If is denoted as v . The viscosity of a fluid depends upon its intermolecular structure. In gases, the molecules are widely spaced resulting in a negligible intermolecular cohesion, while in liquids the molecules being very close to each other, the cohesion is much larger with the increases of temperature, the cohesive force decreases rapidly resulting in the decreases of viscosity. In case of gases, the viscosity is mainly due to transfer of molecular momentum in the transerve direction brought about by the molecular agitation. Molecular agitation increases with rise in temperature. Thus we conclude that viscosity of a fluid may thus be considered to be composed of two parts, first due to intermolecuar cohesion and second due to transfer of molecular momentum. Viscosity of liquids
The general motion of a rigid body can be considered to be a combination of (i) a motion of its centre of mass about an axis, and (ii) its motion about an instantaneous exis passing through the centre of mass. These axes need not be stationary. Consider, for example, a thin uniform disc welded (rigidly fixed) horizontally at its rim to a massless, stick as shown in the figure. When the disc-stick system is rotated about the origin on a horizontal frictionless plane with angular speed omega the motion at any instant can be taken as a combination of (i) a rotation of the disc through an instantaneous vertical axis passing through its centre of mass (as is seen from the changed orientation of points P and Q). Both these motions have the same angular speed omega in this case Now consider two similar system as shown in the figure: Case (a) the disc with its face vertical and parallel to x-z plane, Case (b) the disc with its face making an angle of 45^@ with x-y plane and its horizontal diameter parallel to x-axis. In both the cases, the disc is welded at point P, and the systems are rotated with constant angular speed omega about the z-axis. Which of the following statements regarding the angular speed about the instantaneous axis (passing through the centre of mass) is correct?
The general motion of a rigid body can be considered to be a combination of (i) a motion of its centre of mass about an axis, and (ii) its motion about an instantaneous exis passing through the centre of mass. These axes need not be stationary. Consider, for example, a thin uniform disc welded (rigidly fixed) horizontally at its rim to a massless, stick as shown in the figure. When the disc-stick system is rotated about the origin on a horizontal frictionless plane with angular speed omega the motion at any instant can be taken as a combination of (i) a rotation of the disc through an instantaneous vertical axis passing through its centre of mass (as is seen from the changed orientation of points P and Q). Both these motions have the same angular speed omega in this case Now consider two similar system as shown in the figure: Case (a) the disc with its face vertical and parallel to x-z plane, Case (b) the disc with its face making an angle of 45^@ with x-y plane and its horizontal diameter parallel to x-axis. In both the cases, the disc is welded at point P, and the systems are rotated with constant angular speed omega about the z-axis. . Which of the following statements about the instantaneous axis (passing through the centre of mass) is correct?
A cyclotron is device for accelerating ions and charged particles. It was developed by Lawerence in 1932. The heart of the appritus consists of a split metal pillbox. Figure sHOws top and front views of the halves called dees. A rapidly oscilating potential difference is applied between the Dees. This produces an oscilating electric field in the gap between the dees, the region inside each dee being essentially free of electric field. The Dees are enclosed in an evaccutaed container, and the entire unit is placed in a uniform magnetic field B whose direction is normal to the plane Of Dees, A charged particle of mass 'm' and charge 'q' in the gap between the dees, it moves with constant speed in a semi-circle. The period of uniform circular motion is T=(2pim)/(qB) and is independant of speed. If the time-period of the oscilating elctric field is equal to this time, then the charged particle will be accelerated again and again Answer the following questions (consider the mass of particles remains constant during motion): A cyclotron is accelerating deutrons having mass= 2xx1.6xx10^(-27) kg and charge +e. If B=2T, then the required angular frequency of oscilating electric field is :
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Consider the following statements and arrange in order of true/false a...
Text Solution
|
- The vapour pressure of two miscible liquids A and B are 300 and 500 mm...
Text Solution
|
- Consider the following system. Three different aqueous solution each...
Text Solution
|
- 2.25 g of a Non volatile substance dissolved in 250 g of C6H6.This sol...
Text Solution
|
- Which of following statements are incorrect about Henry's law ?
Text Solution
|
- In which of the following pairs of solutions will the values of the va...
Text Solution
|
- V.P. of solute containing 6gm of non volatile solute in 180gm of water...
Text Solution
|
- Two liquids A and B form an ideal solution.The solution has a vapour p...
Text Solution
|
- Statement-1 : Perfectly ideal solution is not possible with respect to...
Text Solution
|
- Statement-1 : When a cell is placed in hypertonic solution, it shrinks...
Text Solution
|
- Statement-1 : The difference in the boiling points of equimolar soluti...
Text Solution
|
- Statement-1 : When 'a' ML of a 0.1 molal urea solution is mixed with a...
Text Solution
|
- A mixture of NO2 and N2O4 has a vapour density of 38.3 at 300K. What i...
Text Solution
|
- How many unpaired electrons are there in the electronic structure for ...
Text Solution
|
- Which of the following is not a member of 3d-transition series ?
Text Solution
|
- The pressure of two pure liquid A and B which form an ideal solutions ...
Text Solution
|
- The pressure of two pure liquid A and B which form an ideal solutions ...
Text Solution
|
- The pressure of two pure liquid A and B which form an ideal solutions ...
Text Solution
|
- Colligative property measurement is one of the techniques used in the ...
Text Solution
|
- Colligative property measurement is one of the techniques used in the ...
Text Solution
|