A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- At a certain temperature, the first order rate constant k(1) is found ...
Text Solution
|
- In the formation of HBr form H(2) and Br(2), following mechanism is ob...
Text Solution
|
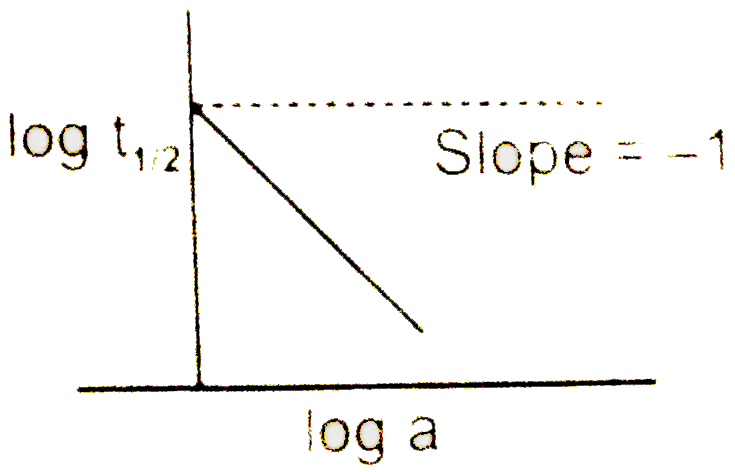
- A graph between log t((1)/(2)) and log a (abscissa), a being the init...
Text Solution
|
- A gaseous reaction A(2)(g) rarr B(g) + (1)/(2) C(g) shows increase in ...
Text Solution
|
- For a 1^(st) order reaction (gaseous) (constant V, T) : aAto(b-1)B+1...
Text Solution
|
- For a certain reaction the variation of rate constant with temperature...
Text Solution
|
- A reaction takes place in theee steps: the rate constant are k(1), k(2...
Text Solution
|
- In a certain reaction, 10% of the reactant decomposes in one hour, 20%...
Text Solution
|
- How many number of unpaired electrons are present in K^(+) ?
Text Solution
|
- Compounds A and B react with a common reagent with first order kinetic...
Text Solution
|
- The activity per ml of a solution of radioactive substances is x.How m...
Text Solution
|
- A reaction with respect to X is zero order till the concentration is r...
Text Solution
|
- For the decomposition of H2O2(aq) it was found that V(O2) (t=15 min) w...
Text Solution
|
- How many number of unpaired electrons are present in Ca^(2+) ?
Text Solution
|
- manganese metal has the electronic configuration [Ar]3d^4 in its +3...
Text Solution
|
- If the rate of reaction, 2SO2(g)+O2(g)overset(Pt)to2SO3(g) is given by...
Text Solution
|
- Which is correct graph :
Text Solution
|
- For a certain reaction Ato products, the t(1//2) as a function of [A]0...
Text Solution
|
- For the reaction 2A+BtoC with the rate low (d[C])/(dt)=k[A]^(1)[B]^(-1...
Text Solution
|
- The number of moles of nitrogen present in one litre of air containing...
Text Solution
|