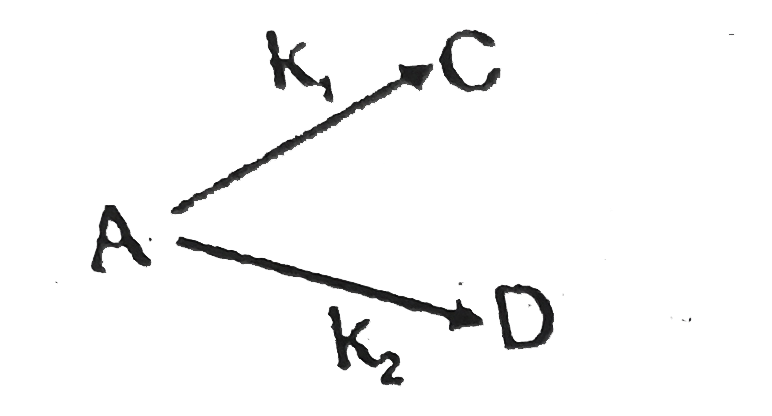
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Elements having complete filled outermost s and p orbital are known as...
Text Solution
|
- A certain reaction A rarr B follows the given concentration (Molarity)...
Text Solution
|
- Consider the following case of competing 1^(st) order reactions. ...
Text Solution
|
- Decomposition of 3A(g)to2B(g)+2C(g) follows first order kinetics.Initi...
Text Solution
|
- Assertion (A) : If the activation energy of a reaction is zero, temper...
Text Solution
|
- Statement-1: For A+2BtoC(rate =K[A]^(1)[B]^(0)), the half life time of...
Text Solution
|
- For I^(st) order decomposition of SO2Cl2(g), SO2Cl2(g)toSO2(g)+Cl2(g...
Text Solution
|
- A mixture of NO2 and N2O4 has a vapour density of 38.3 at 300K. What i...
Text Solution
|
- At N.T.P the volume of a gas is found to be 270mL. What will be the vo...
Text Solution
|
- The initial rate of zero order reaction of the gaseous equation A(g)to...
Text Solution
|
- The variation of concentration of 'A' with time in two experiments sta...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- At N.T.P the volume of a gas is found to be 237mL. What will be the vo...
Text Solution
|
- At N.T.P the volume of a gas is found to be 250mL. What will be the vo...
Text Solution
|
- At N.T.P the volume of a gas is found to be 150mL. What will be the vo...
Text Solution
|
- For a hypotherical elementary reaction where k1/k2=1/2 Initailly on...
Text Solution
|
- For a hypotherical elementary reaction where k1/k2=1/2 Initailly on...
Text Solution
|
- For a hypotherical elementary reaction where k1/k2=1/2 Initailly on...
Text Solution
|