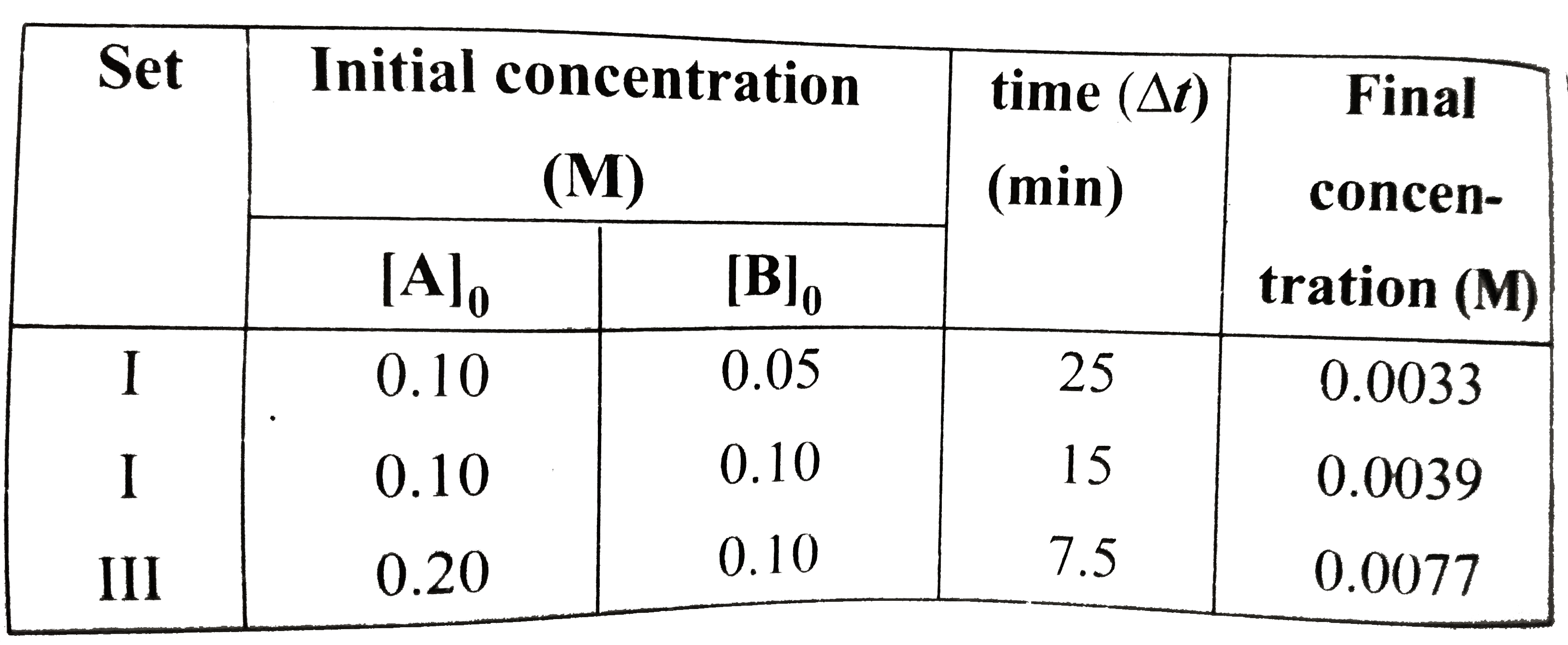
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- The initial rate of zero order reaction of the gaseous equation A(g)to...
Text Solution
|
- The variation of concentration of 'A' with time in two experiments sta...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- At N.T.P the volume of a gas is found to be 237mL. What will be the vo...
Text Solution
|
- At N.T.P the volume of a gas is found to be 250mL. What will be the vo...
Text Solution
|
- At N.T.P the volume of a gas is found to be 150mL. What will be the vo...
Text Solution
|
- For a hypotherical elementary reaction where k1/k2=1/2 Initailly on...
Text Solution
|
- For a hypotherical elementary reaction where k1/k2=1/2 Initailly on...
Text Solution
|
- For a hypotherical elementary reaction where k1/k2=1/2 Initailly on...
Text Solution
|
- At N.T.P the volume of a gas is found to be 270mL. What will be the vo...
Text Solution
|
- At N.T.P the volume of a gas is found to be 240mL. What will be the vo...
Text Solution
|
- Match order of the reaction (in List-I) with the corresponding rate co...
Text Solution
|
- The number of moles of nitrogen present in one litre of air containing...
Text Solution
|
- The number of unpaired electrons in Zn^(2+) is
Text Solution
|
- Graph between log k and 1//T [k rate constant (s^(-1)) and T and the t...
Text Solution
|
- For the reaction Ato products, the following data is given for a parti...
Text Solution
|
- If (dx)/(dt)=k[H(3)O^(+)]n and rate becomes 100 times when pH change...
Text Solution
|
- The gas phase decomposition of dimethyl ether follows first order kine...
Text Solution
|