A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- State the number of unpaired electrons in Ni^(2+) :
Text Solution
|
- Write general configuration of inner transition element. what is the i...
Text Solution
|
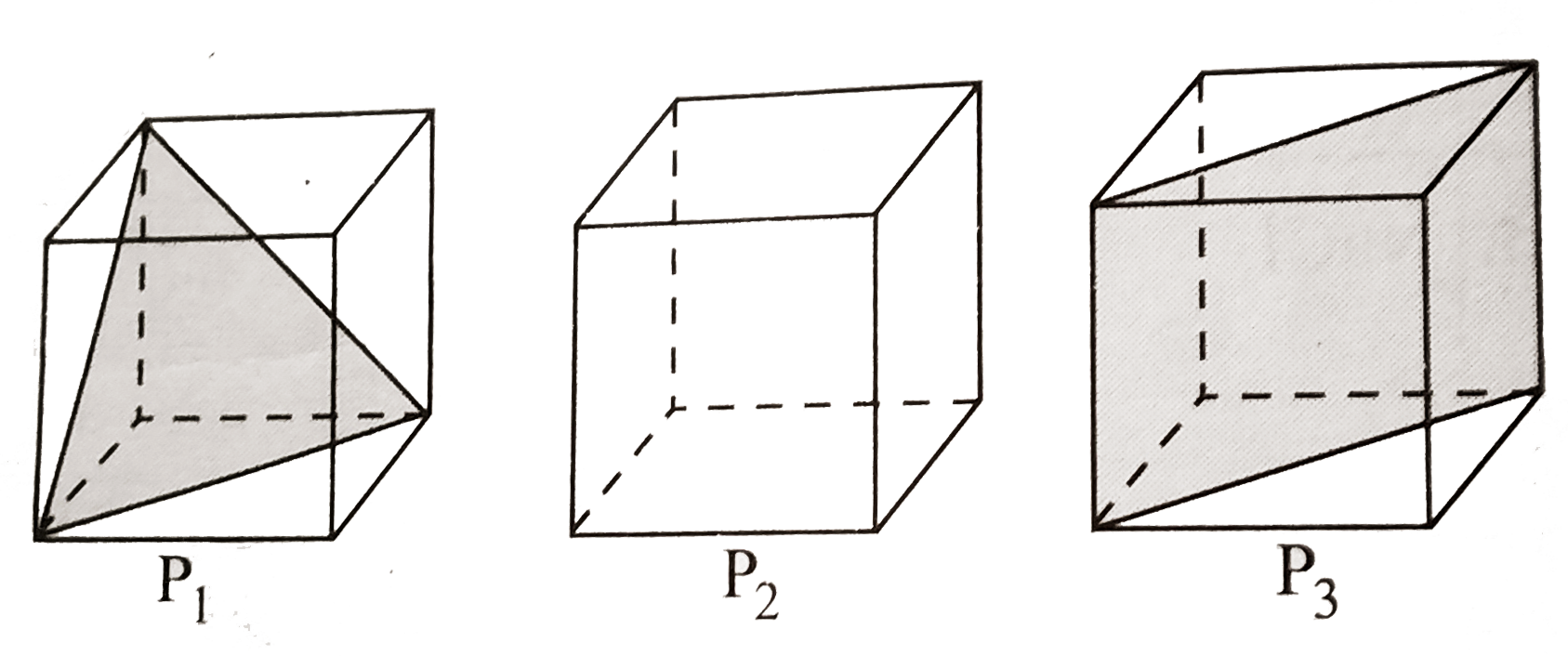
- Following three planes (P(1), P(2), P(3)) in an fcc unit cell are show...
Text Solution
|
- In a AB unit cell (Rock salt type) assuming A^(+) forming fcc :
Text Solution
|
- In the fluorite structure if the radius ratio is (sqrt(3/(2))-1) how m...
Text Solution
|
- The co-ordination number of FCC structure for metals is 12, since:
Text Solution
|
- Select the correct statement(s) related to hexagonal close packing of ...
Text Solution
|
- An hcp and a ccp structure for a given element would be expected to ha...
Text Solution
|
- Assertion : An important feature of fluorite structure is that cations...
Text Solution
|
- Statement-1 : In ZnS zinc blende structure Zn^(2+) from FCC while alte...
Text Solution
|
- Statement-1 : The ratio of Zn^2+ and F^(-) per unit cell of ZnS and Ca...
Text Solution
|
- Assertion :In NaCIcrystal each Na^(+) ion is tourching 6 CI^(-) ion bu...
Text Solution
|
- In hexagonal systems of crystals, a frequently encountered arrangement...
Text Solution
|
- In a Carius determination, 0.25g of an organic substance gave 0.24g of...
Text Solution
|
- The type of seciconductor shown by crystal capable of showing Schotlky...
Text Solution
|
- When an atom or an ion is missing from its nomal lattice site a lattic...
Text Solution
|
- When an atom or an ion is missing from its normal lattice site, a latt...
Text Solution
|
- Only those atoms which four covalent bonds produce a repetitive three ...
Text Solution
|
- Only those atoms which four covalent bonds produce a repetitive three ...
Text Solution
|
- Only those atoms which four covalent bonds produce a repetitive three ...
Text Solution
|