A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Statement-1 : The ratio of Zn^2+ and F^(-) per unit cell of ZnS and Ca...
Text Solution
|
- Assertion :In NaCIcrystal each Na^(+) ion is tourching 6 CI^(-) ion bu...
Text Solution
|
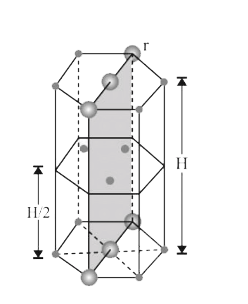
- In hexagonal systems of crystals, a frequently encountered arrangement...
Text Solution
|
- In a Carius determination, 0.25g of an organic substance gave 0.24g of...
Text Solution
|
- The type of seciconductor shown by crystal capable of showing Schotlky...
Text Solution
|
- When an atom or an ion is missing from its nomal lattice site a lattic...
Text Solution
|
- When an atom or an ion is missing from its normal lattice site, a latt...
Text Solution
|
- Only those atoms which four covalent bonds produce a repetitive three ...
Text Solution
|
- Only those atoms which four covalent bonds produce a repetitive three ...
Text Solution
|
- Only those atoms which four covalent bonds produce a repetitive three ...
Text Solution
|
- Column-I and Column-II contains four entries each.Entries of Column-I ...
Text Solution
|
- {:("Column-I","Column-II"),((A)"74% occupancy of space",(p)"cubic clos...
Text Solution
|
- A(2)B molecules(" molar mass " = 259.8 g//"ml") crystallises in a hex...
Text Solution
|
- NA spheres of radius 'R' are melted in x xxNA smaller spheres, so that...
Text Solution
|
- Total number of faces in a truncated octahedron=x Total number of fa...
Text Solution
|
- An ionic compound MCl is simultaneously doped with 10^(-6) mole % NCl2...
Text Solution
|
- Explain the term transuranic elements?
Text Solution
|
- Ionic solid B^+A^(-) crystallizes in rock salt type of structure.1.296...
Text Solution
|
- The arrangement of elements in a group of three was done by Döbereiner...
Text Solution
|
- In F.C.C. arrangement of identical spheres, distance between two neare...
Text Solution
|