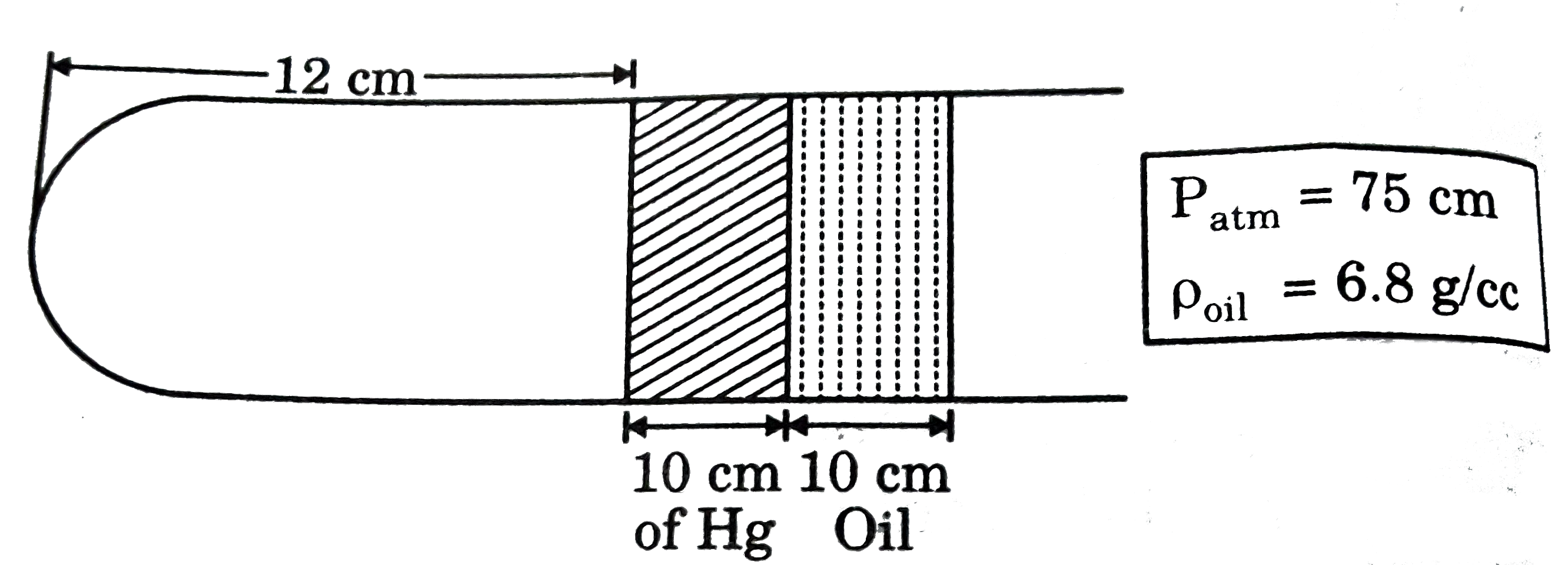
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- How many moles are represented by 120 g of glucose
Text Solution
|
- Consider the following figure at 500 K.Assuming ideal gas behaviour, c...
Text Solution
|
- If above tube is placed vertically with the open and upward then find ...
Text Solution
|
- In a tyre of "Ferrari" car of Mr. Obama, a tube having a volume of 12....
Text Solution
|
- 2 moles of Ne gas and 5 moles of He gas, both samples having average v...
Text Solution
|
- Two glass bulbs of equal volume are connected by a narrow tube and are...
Text Solution
|
- According to Maxwell's distribution of molecular speeds, the following...
Text Solution
|
- A bulb of constant volume is attached to a manometer tube open at othe...
Text Solution
|
- The pressure exerted by 12 g of an ideal gas at temperature T^(@)C in...
Text Solution
|
- A 4: 1 molar mixture of He and CH4 is contained in a vessel at 20 bar ...
Text Solution
|
- What mass of potassium chlorate (KClO3) on heating gives 1.691 g of po...
Text Solution
|
- The number of moles present in 98 g of HSO is
Text Solution
|
- What mass of potassium chlorate (KClO3) on heating gives 1.791 g of po...
Text Solution
|
- The wave motion of electron in a Bohr's orbit of hydrogen is as shown ...
Text Solution
|
- For a hypothetical H like atom which follows Bohr's model, some spectr...
Text Solution
|
- The number of photons emitted in 10 hours by a 60 W sodium lamp (lambd...
Text Solution
|
- The de broglie wavelength of a tennis ball of mass 60 g moving with a...
Text Solution
|
- The photon emitted due to electronic transition of 5^(th) excited stat...
Text Solution
|
- A proton when accelerated through a potential difference of V volt has...
Text Solution
|
- The ionization energy of a hydrogen atom in terms of Rydberg constant ...
Text Solution
|