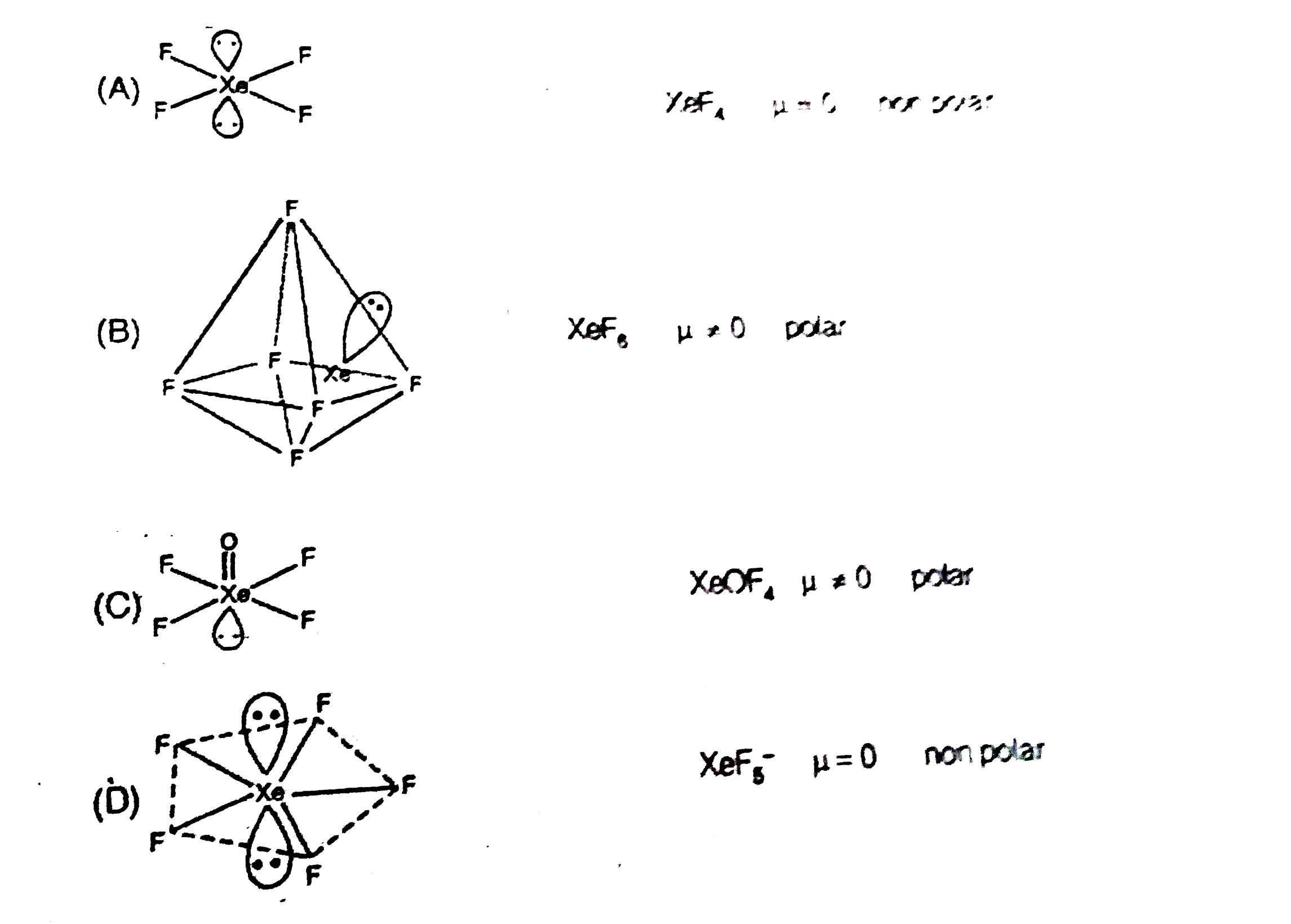A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Select that pairs in which bond angle of first member is higher than s...
Text Solution
|
- Which charge for the N2 molecule would give a bond order of 2.5 ?
Text Solution
|
- 130 mL of pure phosphine is decomposed to produce vapours of phosphoru...
Text Solution
|
- 180 mL of pure phosphine is decomposed to produce vapours of phosphoru...
Text Solution
|
- A flask contain 132g of CO2 gas. Calculate how many moles of CO2 gas d...
Text Solution
|
- A flask contain 13.2g of CO2 gas. Calculate how many moles of CO2 gas ...
Text Solution
|
- A flask contain 17.6g of CO2 gas. Calculate how many moles of CO2 gas ...
Text Solution
|
- In which pair is the stronger bond found in the first species ?
Text Solution
|
- Which of the following statement is/are true ?
Text Solution
|
- How many of g of S are required to produce 10 moles and 10 g of H(2)SO...
Text Solution
|
- How many moles of potassium chlorate need to be heated to produce 11.2...
Text Solution
|
- How many of g of S are required to produce 20 moles and 10 g of H(2)SO...
Text Solution
|
- Assertion : F bond angle p is equal to the bond angle Q but not preci...
Text Solution
|
- Assertion Bond order in a molecule can assume any integral value or fr...
Text Solution
|
- Statement-1:NO^+ and CN^(-) both have same order and magnetism (i.e. m...
Text Solution
|
- Between ionic and covalent bonds, there are large majority of bonds, i...
Text Solution
|
- Between ionic and covalent bonds, there are large majority of bonds, i...
Text Solution
|
- In which of the following mixtures, the London dispersion force acts a...
Text Solution
|
- In the valence bond theory, hybridisation of orbitals is an integral p...
Text Solution
|
- In the valence bond theory, hybridisation of orbitals is an integral p...
Text Solution
|