A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- How many moles of potassium chlorate need to be heated to produce 11.2...
Text Solution
|
- How many of g of S are required to produce 20 moles and 10 g of H(2)SO...
Text Solution
|
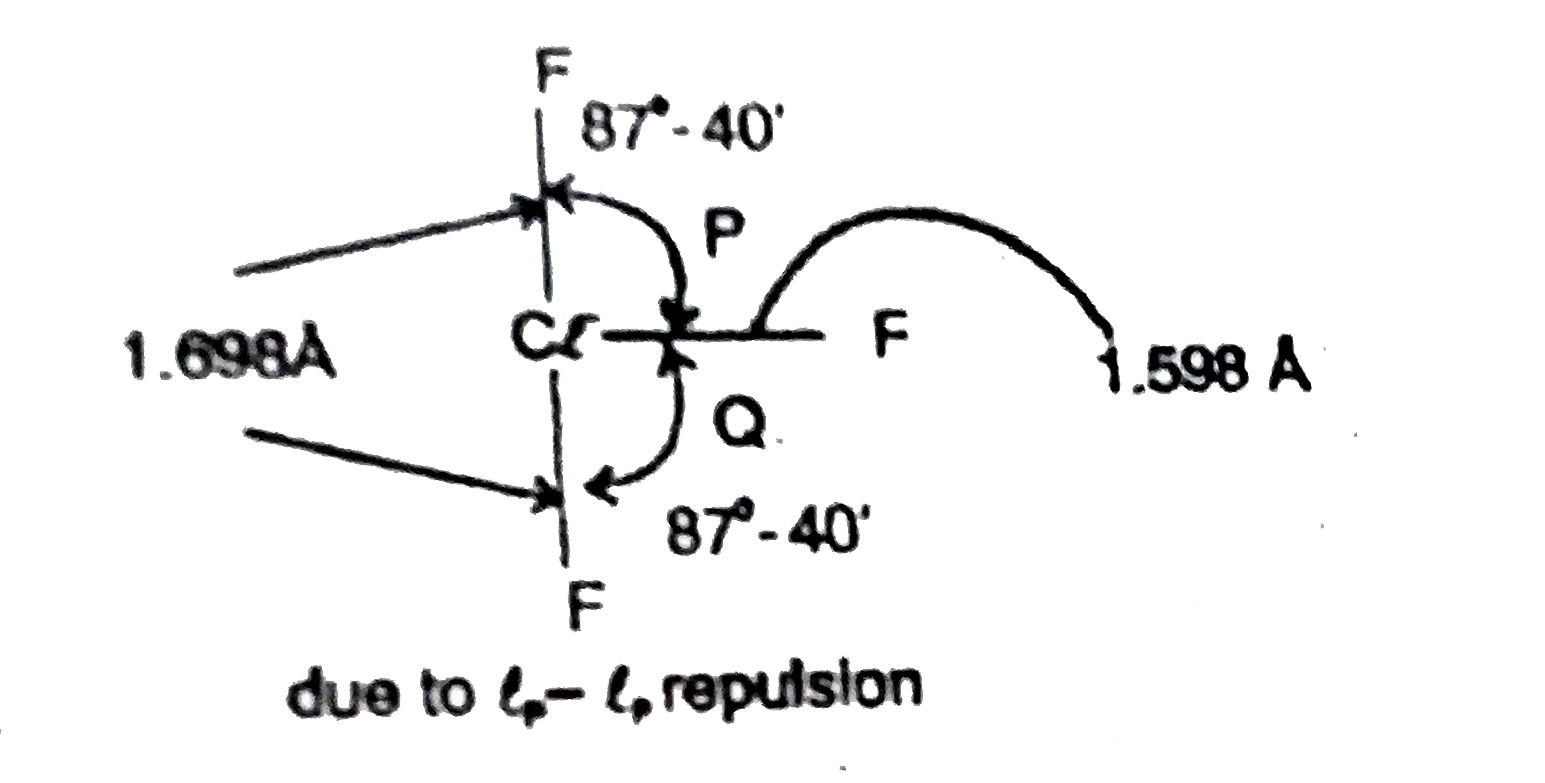
- Assertion : F bond angle p is equal to the bond angle Q but not preci...
Text Solution
|
- Assertion Bond order in a molecule can assume any integral value or fr...
Text Solution
|
- Statement-1:NO^+ and CN^(-) both have same order and magnetism (i.e. m...
Text Solution
|
- Between ionic and covalent bonds, there are large majority of bonds, i...
Text Solution
|
- Between ionic and covalent bonds, there are large majority of bonds, i...
Text Solution
|
- In which of the following mixtures, the London dispersion force acts a...
Text Solution
|
- In the valence bond theory, hybridisation of orbitals is an integral p...
Text Solution
|
- In the valence bond theory, hybridisation of orbitals is an integral p...
Text Solution
|
- In the valence bond theory, hybridisation of orbitals is an integral p...
Text Solution
|
- Nitrogen , oxygen and fluorine are the highly electronegative elements...
Text Solution
|
- Nitrogen , oxygen and fluorine are the highly electronegative elements...
Text Solution
|
- Nitrogen , oxygen and fluorine are the highly electronegative elements...
Text Solution
|
- The distribution of electrons among molecular orbitals is called the e...
Text Solution
|
- Arrange the following molecule/species in the order of their increasi...
Text Solution
|
- N2 has greater bond energy than N(2)^(+) but O2 has lower bond dissoci...
Text Solution
|
- 54 g of sodium chloride is dissolved in 100 g of water at 293 K. Find ...
Text Solution
|
- 100gm of an aq. solution of sugar contains 40% sugar by mass. How much...
Text Solution
|
- 1.49g of ammonia at STP occupies a volume of 2.48 dm^3 calculate the m...
Text Solution
|
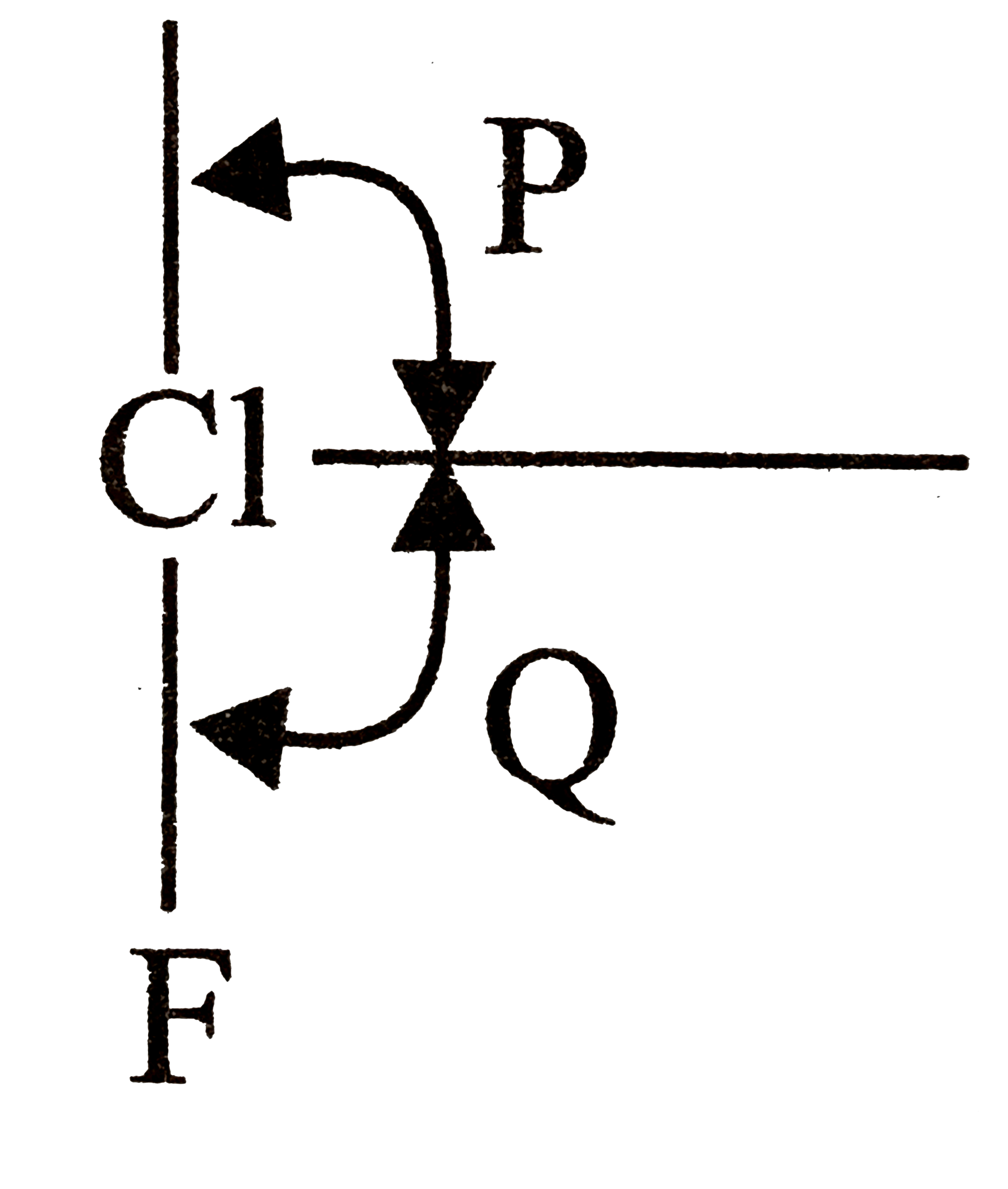 F bond angle p is equal to the bond angle Q but not precisely equal to `90^(@)`.
F bond angle p is equal to the bond angle Q but not precisely equal to `90^(@)`.