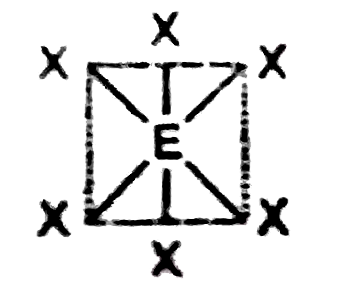
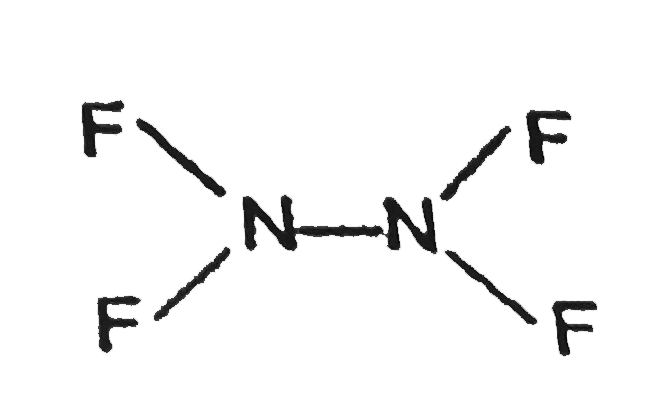
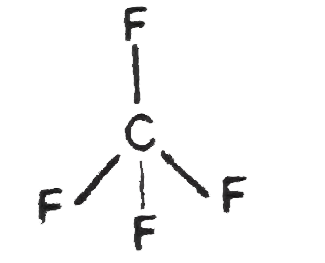Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- How many of g of S are required to produce 10 moles and 20 g of H(2)SO...
Text Solution
|
- How many of the following compounds have significant involvement of d ...
Text Solution
|
- Is the following statements are correct ? (i)Among NH3, PH3, AsH3, a...
Text Solution
|
- The bond order of : NO is
Text Solution
|
- How many maximum atoms lie in the same plane in the structure of methy...
Text Solution
|
- How many of g of S are required to produce 20 moles and 20 g of H(2)SO...
Text Solution
|
- The difference in the number of sigma and pi bond in trimer of SO3 i.e...
Text Solution
|
- In how many following species , the central atoms have two lone pairs ...
Text Solution
|
- BrF3 is a liquid which considerably undergoes self ionization to form ...
Text Solution
|
- How many hydrogen-bonded water molecule(s) are associated in CuSO(4).5...
Text Solution
|
- Sum of antibonding pi electrons (pi electrons ) in species O2, O2^(-) ...
Text Solution
|
- Amongst the following the total number of species which does/do not ex...
Text Solution
|
- If the dipole moment of AB molecule is given by 1.2 D and A - B the bo...
Text Solution
|
- How many oxides are soluble in moderaterly concentrated aqueous soluti...
Text Solution
|
- For anion of oxyacid, formed by removal of one water molecule from Bor...
Text Solution
|
- For anion of CaS2O8 , the only correct combination is :
Text Solution
|
- For given oxidation number in Column-I, the only correct combination f...
Text Solution
|
- Which of the following are bidentate monoanion ligands ? (1) Acetyla...
Text Solution
|
- How many moles of potassium chlorate need to be heated to produce 1.2 ...
Text Solution
|
- The EAN of metal atoms in [Fe(XO)2(NO^+)2] and Co2 (CO)8 respectively ...
Text Solution
|