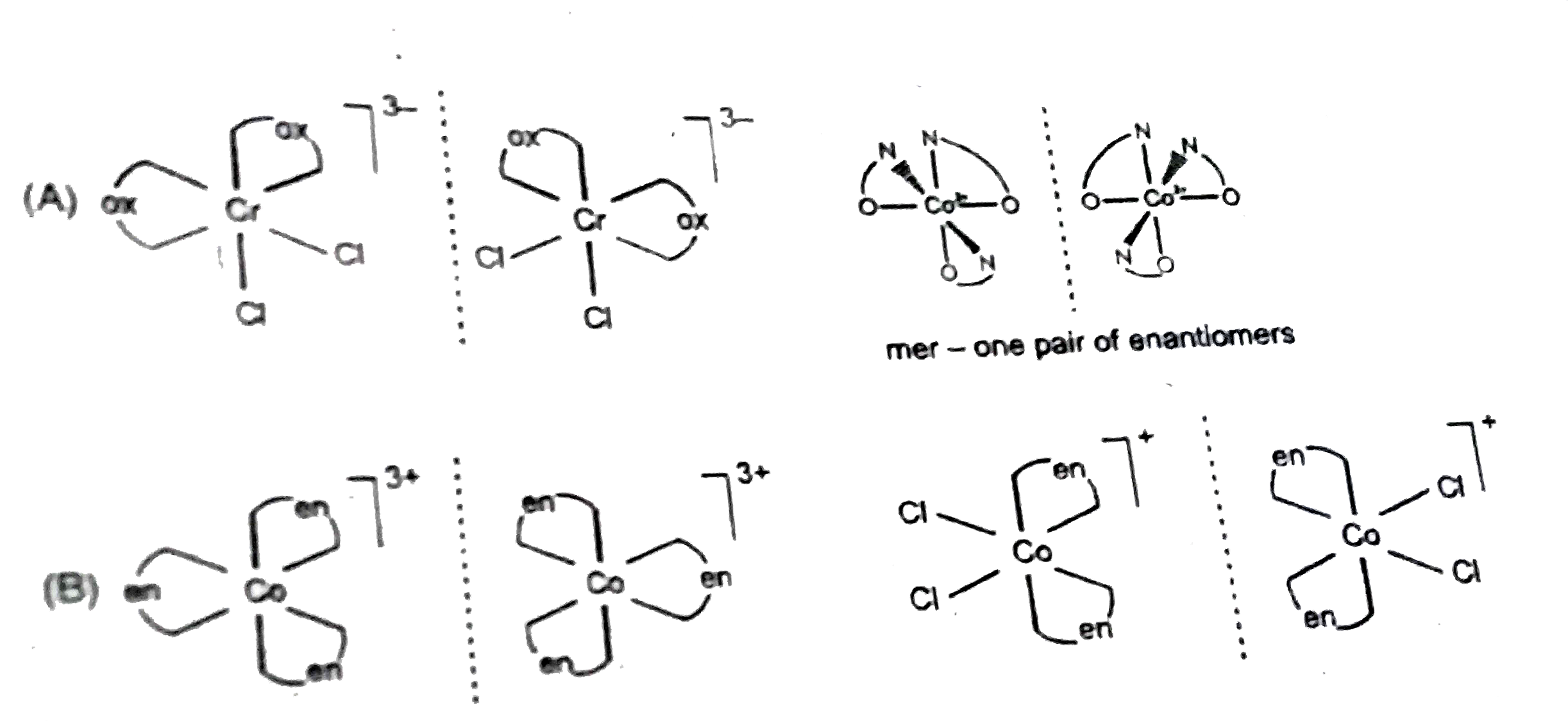
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- How many moles of potassium chlorate need to be heated to produce 18.2...
Text Solution
|
- How many moles of potassium chlorate need to be heated to produce 22.4...
Text Solution
|
- How many of g of S are required to produce 25 moles and 25 g of H(2)SO...
Text Solution
|
- Statement-1:In natural tris chelating complex, [Co(acac)3], in each ri...
Text Solution
|
- The question consist of two statements each, printed as Assertion and ...
Text Solution
|
- Statement-1:Bis(dimethylglyoximato)nickel(II) can show geometrical iso...
Text Solution
|
- The question consist of two statements each, printed as Assertion and ...
Text Solution
|
- The IUPAC name of [Co(H2NCH2CH2NH2)2 (NO2)NO5 is bis(enthane-1,2-diami...
Text Solution
|
- The IUPAC name of [Co(H2NCH2CH2NH2)2 (NO2)NO5 is bis(enthane-1,2-diami...
Text Solution
|
- The IUPAC name of the coordination compound [NICl(NO2)(PPh3)2] is :
Text Solution
|
- In coordination chemistry there are a variety of methods applied to fi...
Text Solution
|
- on reaction with concentrated H2SO4, it suffers loss in weight and on ...
Text Solution
|
- In coordination chemistry there are a variety of methods applied to fi...
Text Solution
|
- Co^(2+)(aq.)+SCN^(-)(aq.)to"Complex"(X) Ni^(2+)(aq.)+"Dimethylglyoxi...
Text Solution
|
- Co^(2+)(aq.)+SCN^(-)(aq.)to"Complex"(X) Ni^(2+)(aq.)+"Dimethylglyoxi...
Text Solution
|
- Co^(2+)(aq.)+SCN^(-)(aq.)to"Complex"(X) Ni^(2+)(aq.)+"Dimethylglyoxi...
Text Solution
|
- 2.49g of ammonia at STP occupies a volume of 4.48 dm^3 calculate the m...
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- Which of the following complexes are diamagnetic ? {:([Pt(NH3)4]^(2+...
Text Solution
|
- In metal carbonyls, the increase in C-O bond length in CO as compared ...
Text Solution
|