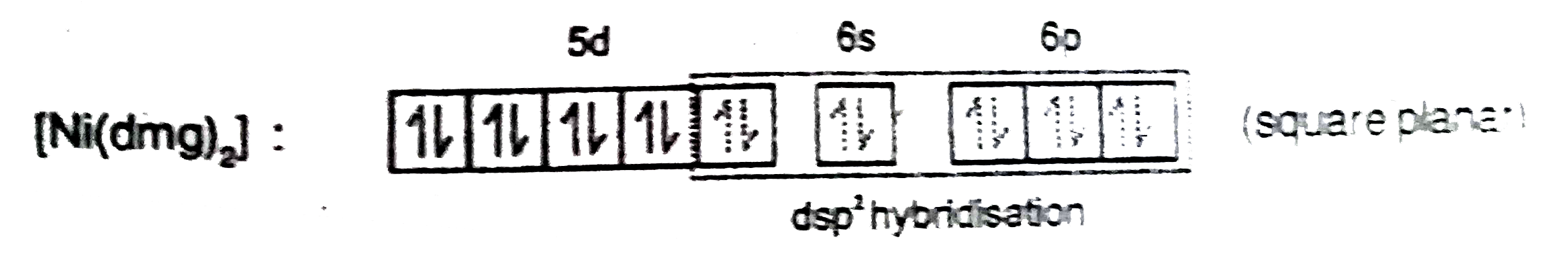A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
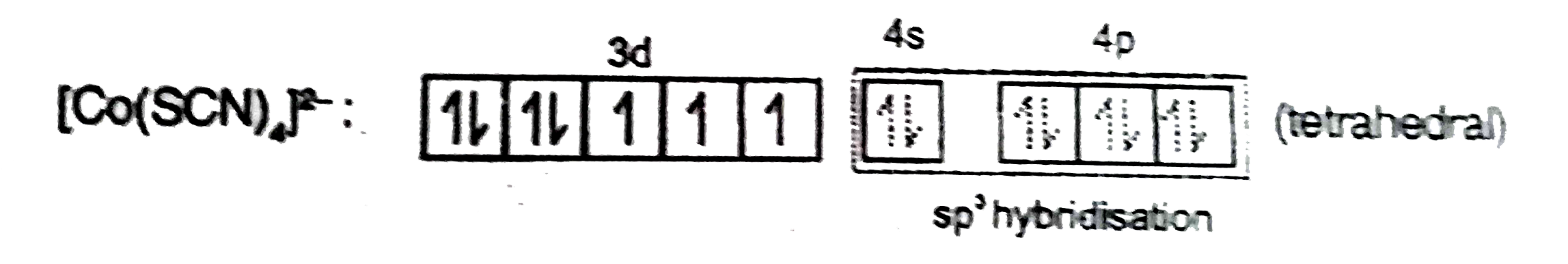
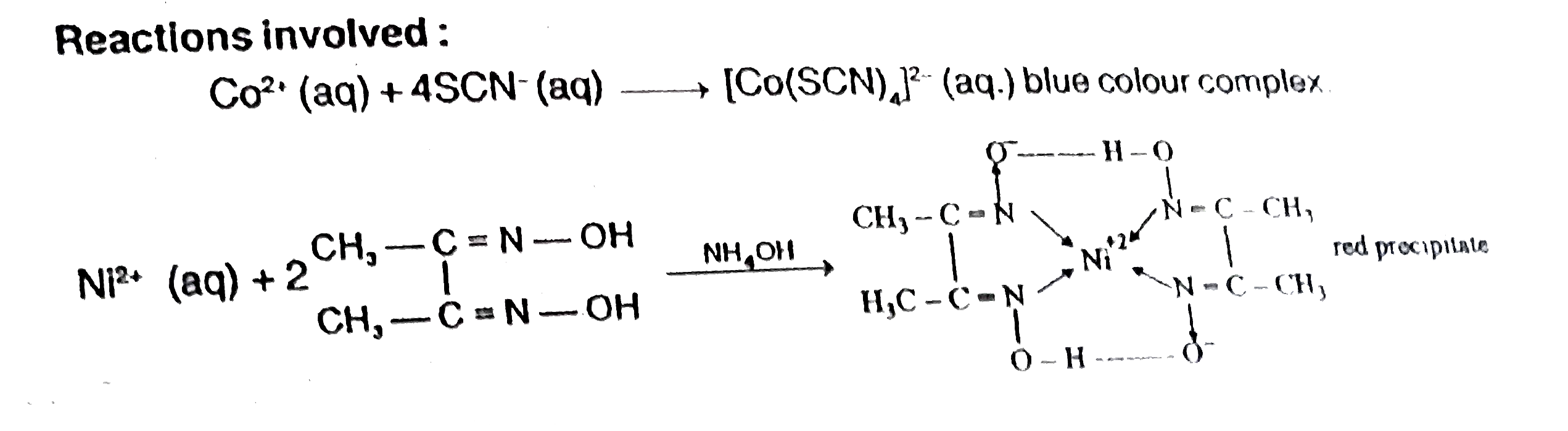
- Co^(2+)(aq.)+SCN^(-)(aq.)to"Complex"(X) Ni^(2+)(aq.)+"Dimethylglyoxi...
Text Solution
|
- Co^(2+)(aq.)+SCN^(-)(aq.)to"Complex"(X) Ni^(2+)(aq.)+"Dimethylglyoxi...
Text Solution
|
- Co^(2+)(aq.)+SCN^(-)(aq.)to"Complex"(X) Ni^(2+)(aq.)+"Dimethylglyoxi...
Text Solution
|
- 2.49g of ammonia at STP occupies a volume of 4.48 dm^3 calculate the m...
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- Which of the following complexes are diamagnetic ? {:([Pt(NH3)4]^(2+...
Text Solution
|
- In metal carbonyls, the increase in C-O bond length in CO as compared ...
Text Solution
|
- Which amongst the following metal carbonyls are inner orbital complexe...
Text Solution
|
- Which one of the following metal carbonyls involves the d^2sp^3 hybrid...
Text Solution
|
- To make a saturated solution, 36 g of sodium chloride is dissolved in ...
Text Solution
|
- The density of a salt solution is 1.13gcm^(−3) and it contains 18% o...
Text Solution
|
- {:("Column-I","Column-II"),((A)[Ni(CO)4],(p)"Octahedral paramagnetic")...
Text Solution
|
- Match the complexed listed column -I with type of hybridisation liste...
Text Solution
|
- The density of a salt solution is 1.13gcm^(−3) and it contains 18% o...
Text Solution
|
- Among the following complexes , how many have 'spin only' magnetic mom...
Text Solution
|
- How many of the following complexes are correctly matched with given p...
Text Solution
|
- Explain with examples what is meant by chelate ligands ?
Text Solution
|
- How many isomers are possible for the complex [Ir(CO)Cl(PPh3)2]?
Text Solution
|
- What is the coordination number of the metal ion in the red complex io...
Text Solution
|
- The brown ring complex compound of iron is formulated as [Fe (H(2)O)(5...
Text Solution
|