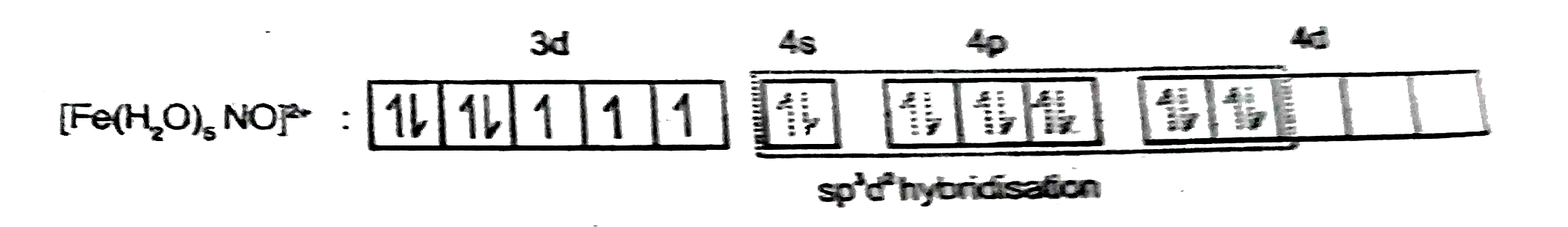Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- The density of a salt solution is 1.13gcm^(−3) and it contains 18% o...
Text Solution
|
- {:("Column-I","Column-II"),((A)[Ni(CO)4],(p)"Octahedral paramagnetic")...
Text Solution
|
- Match the complexed listed column -I with type of hybridisation liste...
Text Solution
|
- The density of a salt solution is 1.13gcm^(−3) and it contains 18% o...
Text Solution
|
- Among the following complexes , how many have 'spin only' magnetic mom...
Text Solution
|
- How many of the following complexes are correctly matched with given p...
Text Solution
|
- Explain with examples what is meant by chelate ligands ?
Text Solution
|
- How many isomers are possible for the complex [Ir(CO)Cl(PPh3)2]?
Text Solution
|
- What is the coordination number of the metal ion in the red complex io...
Text Solution
|
- The brown ring complex compound of iron is formulated as [Fe (H(2)O)(5...
Text Solution
|
- In how many of the following complex ions, the central metal ions use ...
Text Solution
|
- The volume (in mL) of 1.0 M AgNO3 required for complete precipitation ...
Text Solution
|
- Using IUPAC NAME write the formula of 1) Tetrahydroxozincate(II) ...
Text Solution
|
- How many isomers of the complex Pt(NH3)2(SCN)2 are possible ?
Text Solution
|
- How many total isomers including constitutional isomers and stereoisom...
Text Solution
|
- [Cu(NH3)4]^(2+) ion is coloured while [Cu(CN)4]^(3-) ion is colourless...
Text Solution
|
- For a blue coloured solution obtained in column-2, select the only cor...
Text Solution
|
- For colourless solution obtained in column-3 , the incorrect option is...
Text Solution
|
- The density of a salt solution is 1.13gcm^(−3) and it contains 18% o...
Text Solution
|
- 100gm of an aq. solution of sugar contains 40% sugar by mass. How much...
Text Solution
|