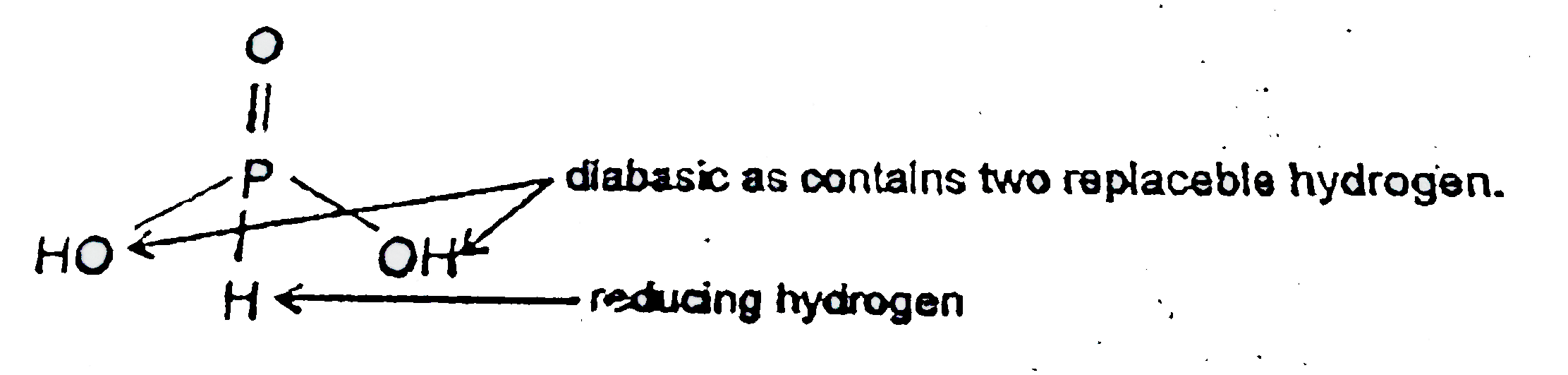A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- The decrease in stability of higher oxidation state in p-block with in...
Text Solution
|
- 15 g of sodium chloride is dissolved in 100 g of water at 293 K. Find ...
Text Solution
|
- For H(3)PO(3) and H(3)PO(4) the correct choice is
Text Solution
|
- One of the products of the following reaction KCNO+(NH4)2SO4oversetDel...
Text Solution
|
- The oxidation states of sulphur in the anions SO(3)^(2-), S(2)O(4)^(2-...
Text Solution
|
- Which of the following option is correctly matched ?
Text Solution
|
- Which of the following statement is incorrect for hydrogen peroxide ?
Text Solution
|
- Sulphuric acid is considered as the king of chemicals. The prosperity ...
Text Solution
|
- Which one of the following statements regarding helium is incorrect ...
Text Solution
|
- {:(F2+dil.NaOHto A+B+C),(F2+conc.NaOHtoA+D+C):} Which is correct?
Text Solution
|
- Ammonia gas can evolve by heating :
Text Solution
|
- Calculate the mass of potassium sulfate required to prepare its 15% so...
Text Solution
|
- Calculate the mass of potassium sulfate required to prepare its 20% so...
Text Solution
|
- Calculate the mass of potassium sulfate required to prepare its 25% so...
Text Solution
|
- Calculate the mass of potassium sulfate required to prepare its 30% so...
Text Solution
|
- Calculate the mass of potassium sulfate required to prepare its 35% so...
Text Solution
|
- Calculate the mass of potassium sulfate required to prepare its 40% so...
Text Solution
|
- Calculate the mass of potassium sulfate required to prepare its 45% so...
Text Solution
|
- Which of the following statements regarding hydrogen peroxide is false...
Text Solution
|
- Sodium thiosulphate is prepared by
Text Solution
|