Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- 3.49g of ammonia at STP occupies a volume of 3.49 dm^3 calculate the m...
Text Solution
|
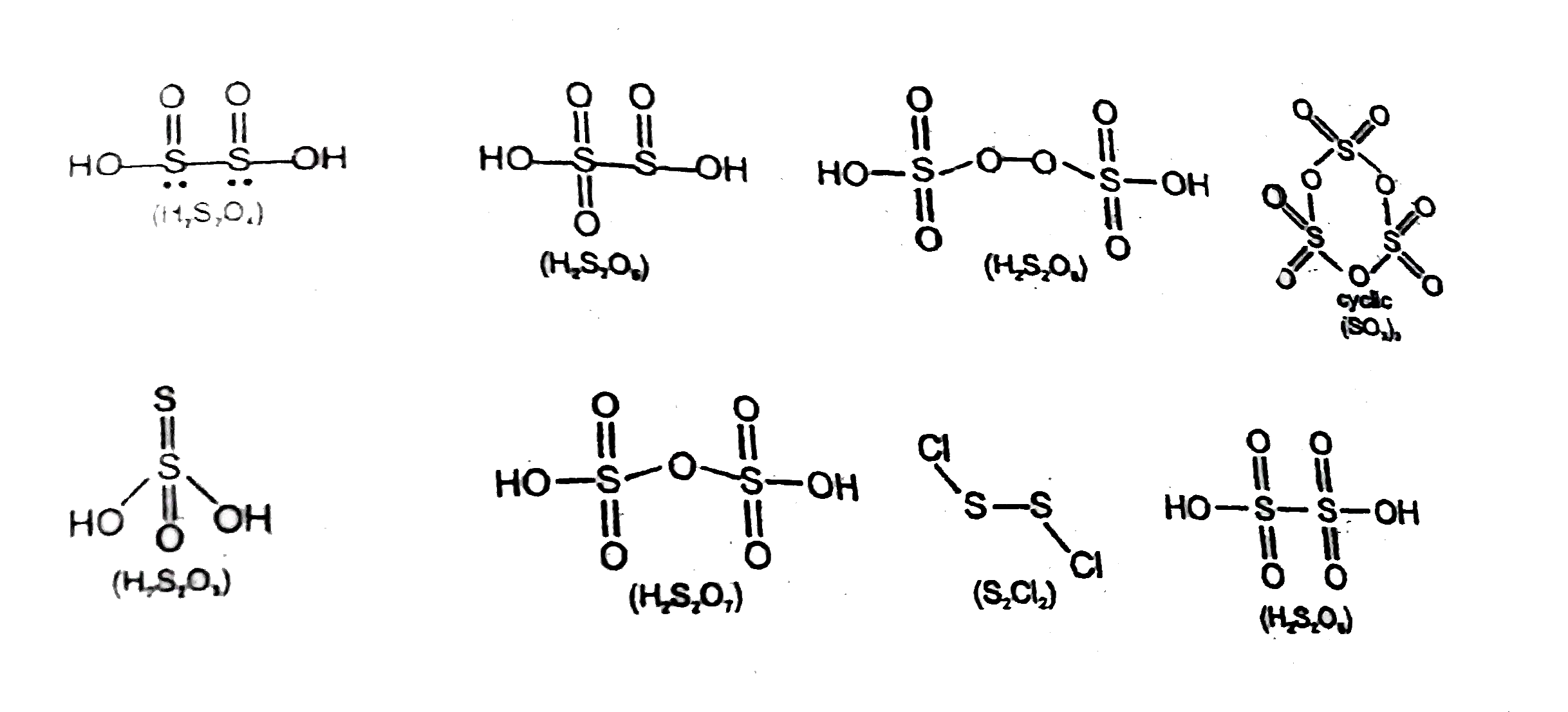
- How many of the following compounds has X-X bonds i.e., single cova...
Text Solution
|
- In how many of the following compounds of sulphur, there is S-S bond (...
Text Solution
|
- If Phosphonic acid, Tetrathionic acid and Pyrophosphoric acid have n...
Text Solution
|
- How many oxygen atoms are shared per SiO4 tetrahedral in Beryl ?
Text Solution
|
- The number of hydrogen atom(s) attached to phosphorus atom in phosphon...
Text Solution
|
- XeF4 disproportionates in water giving reduced and oxidised products W...
Text Solution
|
- What is the value of X in the given chemical formula of crystalline ...
Text Solution
|
- What is the sum of the oxidation state of phosphorus and number of P...
Text Solution
|
- How many moles of phosphine are produced when one of the calcium phosp...
Text Solution
|
- 3.49g of ammonia at STP occupies a volume of 2.48 dm^3 calculate the m...
Text Solution
|
- In how many of the following compounds of sulphur, there is S-S bond (...
Text Solution
|
- How much mass of sodium acetate is required to make 210 mL of 0.575 mo...
Text Solution
|
- Chlorine reacts with dilute NaOH under ordinary conditions to give
Text Solution
|
- How much mass of sodium acetate is required to make 200 mL of 0.575 mo...
Text Solution
|
- How much mass of sodium acetate is required to make 240 mL of 0.575 mo...
Text Solution
|
- In general the melting and boiling points of transition metals
Text Solution
|
- Which of the following is the most suitable description of transition ...
Text Solution
|
- Among the following, the species that is both paramagnetic and coloure...
Text Solution
|
- In which of the following oxyanions the oxidation state of central ato...
Text Solution
|