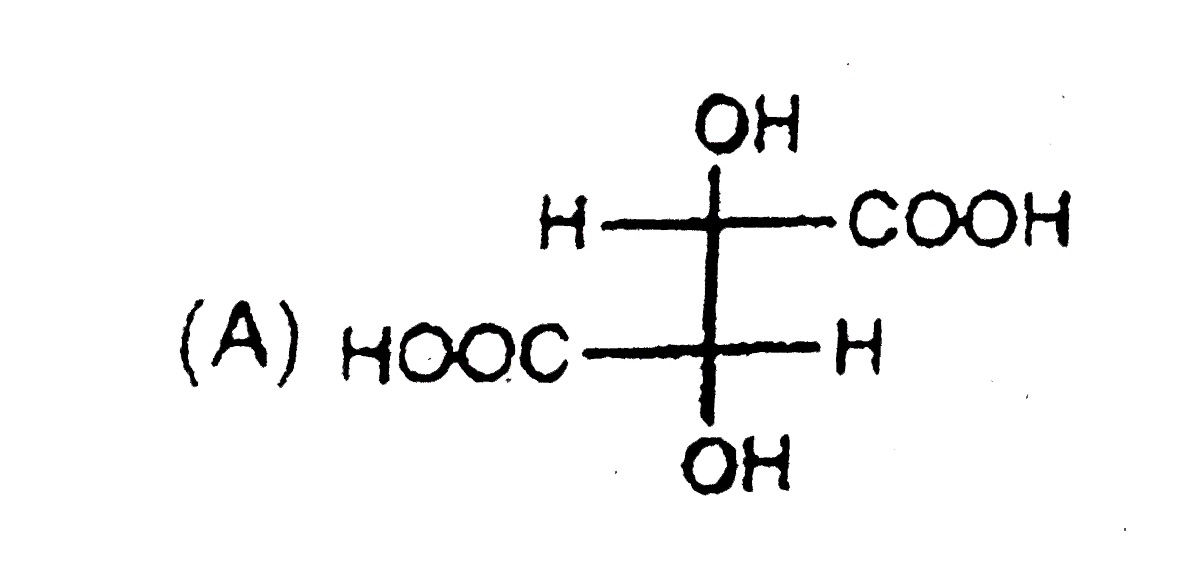
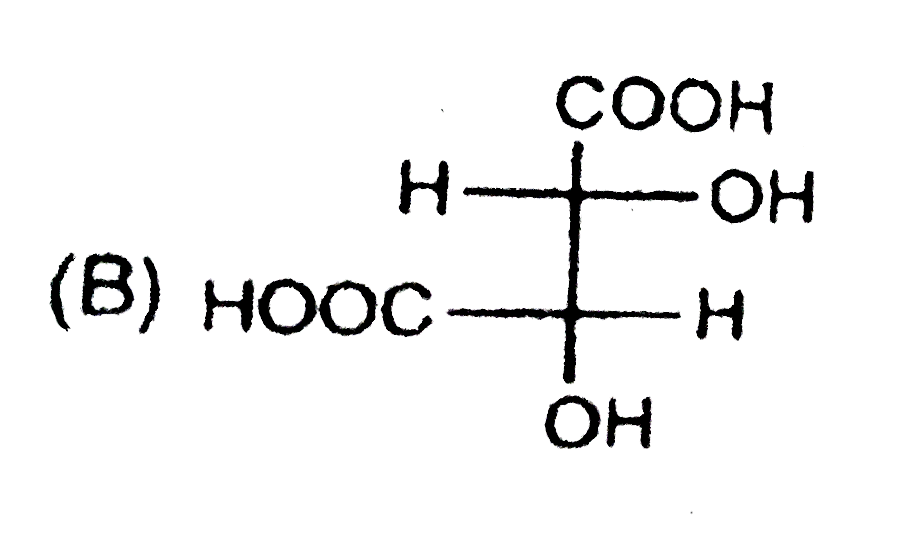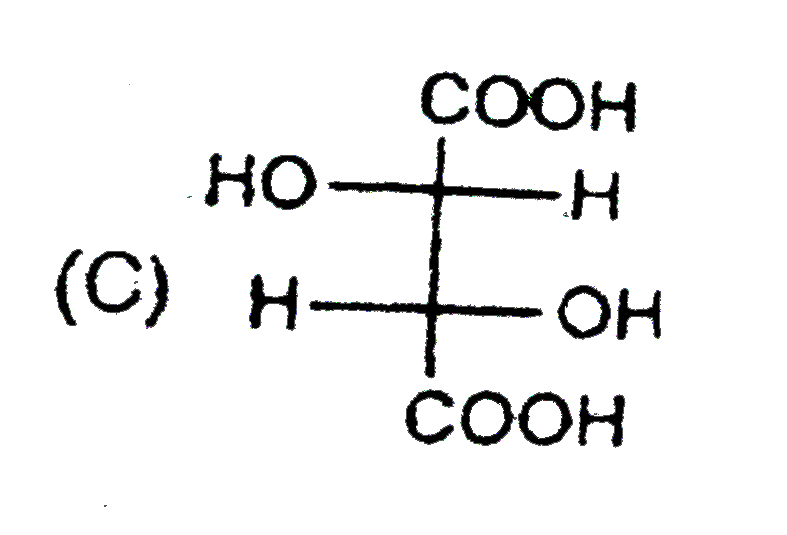A
B
C
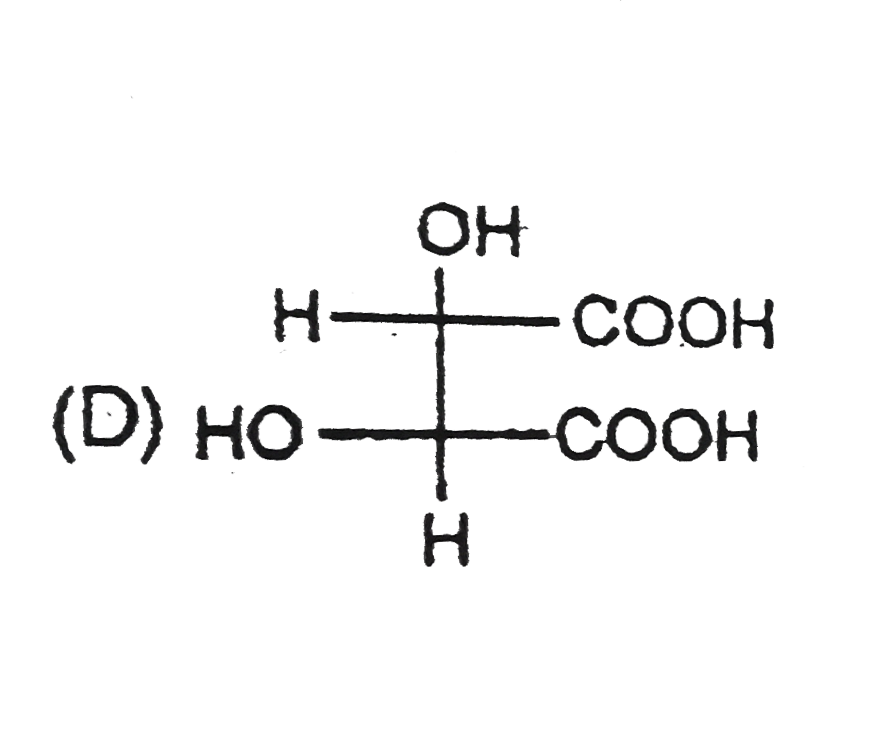
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- How many assymmetric carbon atoms are present in (i)1,2-Dimethyl cyc...
Text Solution
|
- Which functional group is present in a molecule of CH3OH?
Text Solution
|
- The stereochemical formula of diastereomer 'Y' of optically active com...
Text Solution
|
- State the meaning of functional group in a carbon compound. Write the ...
Text Solution
|
- Stibene (PhCH=CHPh). Can exist in two diastereomeric forms (X) and (Y)...
Text Solution
|
- Which of the following is highly inflammable ?
Text Solution
|
- When ethyl bromide is heated with sodium in dry ether solvent, the alk...
Text Solution
|
- does 2 butene compound is showing geometrical isomers:
Text Solution
|
- Methane with the Molecular formula CH4 has
Text Solution
|
- What is the mole fraction of glucose in 40% w/W glucose solution ?
Text Solution
|
- Draw two isomers of Butene and name them.
Text Solution
|
- What is the mole fraction of glucose in 45% w/W glucose solution ?
Text Solution
|
- 3.49g of ammonia at STP occupies a volume of 5.48 dm^3 calculate the m...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 1.5kg of 0.3...
Text Solution
|
- What are the conditions necessary for a compound to show geometrical i...
Text Solution
|
- In a Carius determination, 0.35g of an organic substance gave 0.24g of...
Text Solution
|
- In a Carius determination, 0.15g of an organic substance gave 0.24g of...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 1.5kg of 0.2...
Text Solution
|
- How much mass of sodium acetate is required to make 300 mL of 0.575 mo...
Text Solution
|
- How much mass of sodium acetate is required to make 350 mL of 0.575 mo...
Text Solution
|