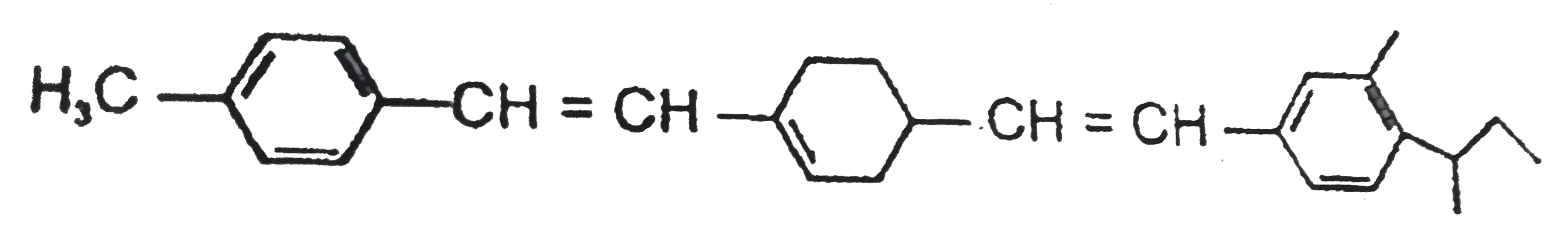
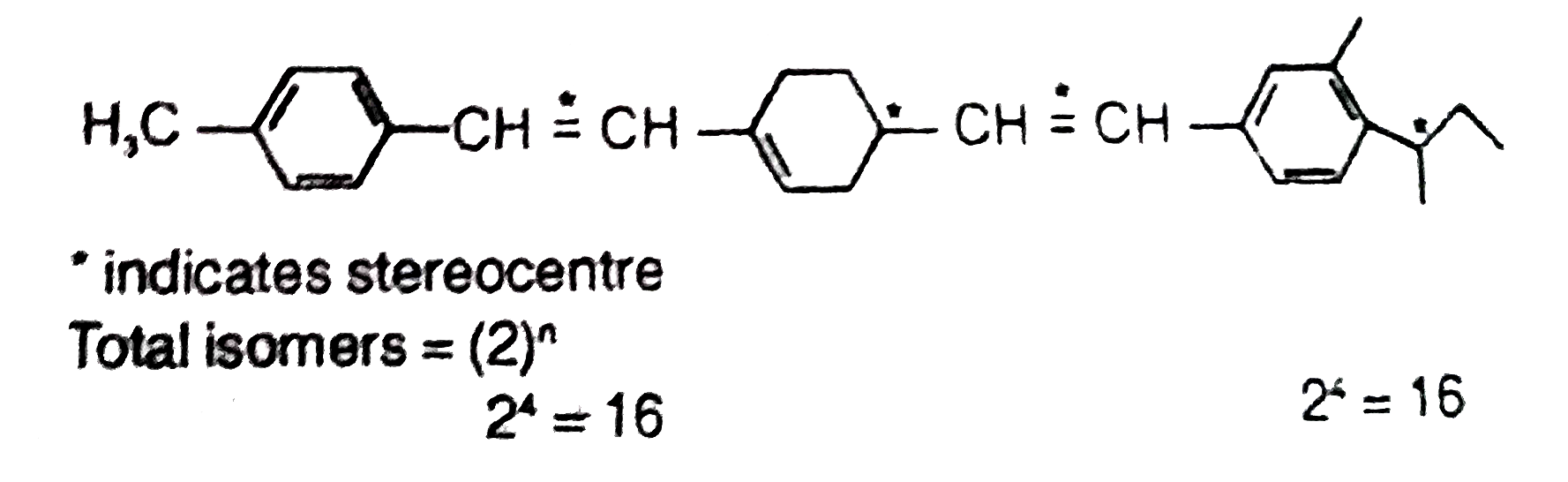
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Cocaine is hallucinogen used widely How many chiral carbon does it hav...
Text Solution
|
- Aspirin is a pain relieving drug.It is an 2-Alkanoyloxybenzoic acid de...
Text Solution
|
- Total number of stereoisomers possible for the given molecule will b...
Text Solution
|
- The number of isomers for the compound with molecule formula C2HDFCl i...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 4.5kg of 0.3...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 2.5kg of 0.4...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 3.5kg of 0.2...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 4.5kg of 0.2...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 5.5kg of 0.2...
Text Solution
|
- The mass of NaCl required to prepare 0.05 m aqueous solution in 1kg wa...
Text Solution
|
- If the number of Chiral carbon in camphor is X and total number of rac...
Text Solution
|
- The mass of NaCl required to prepare 0.04 m aqueous solution in 1kg wa...
Text Solution
|
- Volume of 0.1 M H2SO4 solution required to neutralize 30 ml of 0.1 M ...
Text Solution
|
- Volume of 0.1 M H2SO4 solution required to neutralize 20 ml of 0.1 M ...
Text Solution
|
- Volume of 0.1 M H2SO4 solution required to neutralize 10 ml of 0.1 M ...
Text Solution
|
- Correct order of resonance energy of following compounds :
Text Solution
|
- Guanidine, (NH2)2 C=NH is said to be the strongest nitrogen containing...
Text Solution
|
- The least and most stable resonating structure respectively are :
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 2.5kg of 0.3...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 3.5kg of 0.3...
Text Solution
|