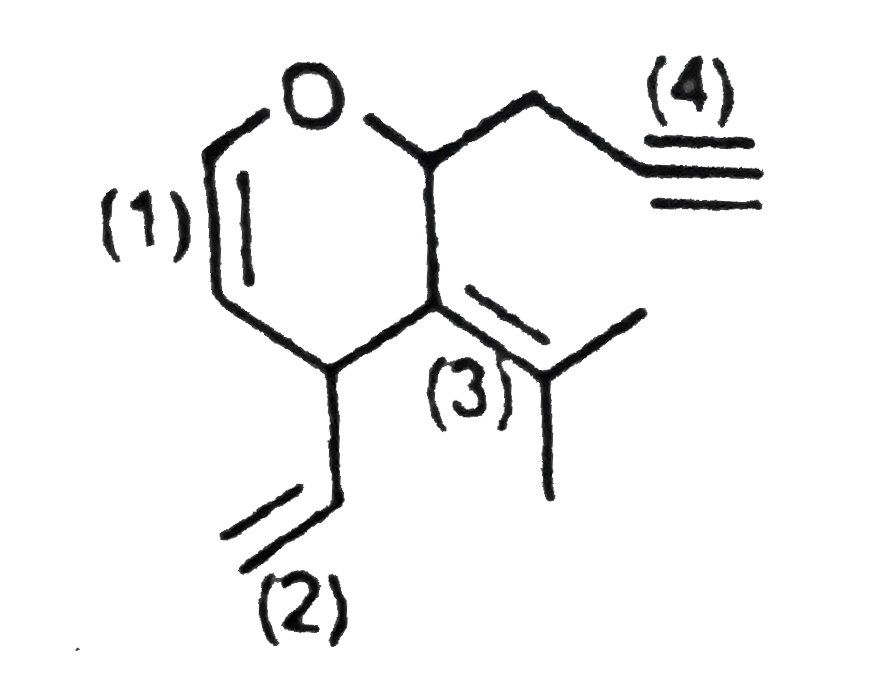
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Volume of 0.1 M H2SO4 solution required to neutralize 60 ml of 0.1 M ...
Text Solution
|
- Volume of 0.1 M H2SO4 solution required to neutralize 70 ml of 0.1 M ...
Text Solution
|
- The correct reactivity order of following C=C/C=C bonds towards Br^(o+...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 1.5kg of 0.4...
Text Solution
|
- The correct order of acidic strength is :
Text Solution
|
- The mass of NaCl required to prepare 0.09 m aqueous solution in 1kg wa...
Text Solution
|
- The mass of NaCl required to prepare 0.1 m aqueous solution in 1kg wat...
Text Solution
|
- The mass of NaCl required to prepare 0.2 m aqueous solution in 1kg wat...
Text Solution
|
- The correct basicity order of atoms p,q and r is :
Text Solution
|
- The correct basic strength order is :
Text Solution
|
- Volume of 0.1 M H2SO4 solution required to neutralize 20 ml of 0.2 M ...
Text Solution
|
- Which of the following statements regarding resonance is NOT correct?
Text Solution
|
- Volume of 0.1 M H2SO4 solution required to neutralize 40 ml of 0.2 M ...
Text Solution
|
- Volume of 0.1 M H2SO4 solution required to neutralize 10 ml of 0.2 M ...
Text Solution
|
- Choose the INCORRECT statement :
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 3.5kg of 0.4...
Text Solution
|
- The mass of NaCl required to prepare 0.3 m aqueous solution in 1kg wat...
Text Solution
|
- Find the mole fraction of solute in its 3 molal aqueous solution.
Text Solution
|
- Purine has four nitrogen's , which of the following are you expect to ...
Text Solution
|
- What is true regarding the relative basic character of the following p...
Text Solution
|