A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- The mass of NaCl required to prepare 0.3 m aqueous solution in 1kg wat...
Text Solution
|
- Find the mole fraction of solute in its 3 molal aqueous solution.
Text Solution
|
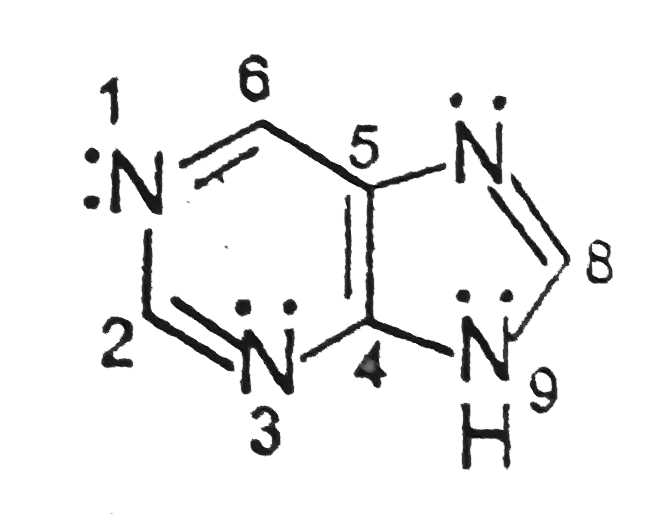
- Purine has four nitrogen's , which of the following are you expect to ...
Text Solution
|
- What is true regarding the relative basic character of the following p...
Text Solution
|
- Which of the following compound can not reacts with hydroxylamine ?
Text Solution
|
- Statement-1:Ortho iodobenzoic acid is strongest acid among all ortho h...
Text Solution
|
- Assertion: Salicyclic acid is much strogest than its m-p-isomers and...
Text Solution
|
- The concept of resonance explains various properties of compounds.The ...
Text Solution
|
- Calculate the mass of urea (NH2CONH2) required in making 5.5kg of 0.4...
Text Solution
|
- The mass of NaCl required to prepare 0.4 m aqueous solution in 1kg wat...
Text Solution
|
- Which of the following shows configurationally isomerism ?
Text Solution
|
- Amines are derivatives of ammonia and are classified as 1^@, 2^@ , and...
Text Solution
|
- Amines are derivatives of ammonia and are classified as 1^@, 2^@ , and...
Text Solution
|
- Match the contents of Column I with the contents of Column II
Text Solution
|
- Match the pKa values with the given compounds
Text Solution
|
- Observe the following compound and write the number of hydrogen atom i...
Text Solution
|
- Value of x used for completion of reaction will be :
Text Solution
|
- The number of hyperconjuable hydrogen atom in the compound is :
Text Solution
|
- In how many species positive charge is not delocalized
Text Solution
|
- How many carboncations are more stable than benzycarbocation Ph-Covers...
Text Solution
|