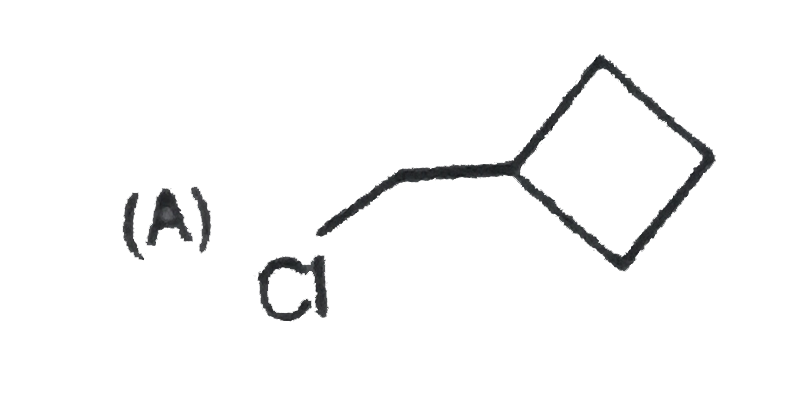
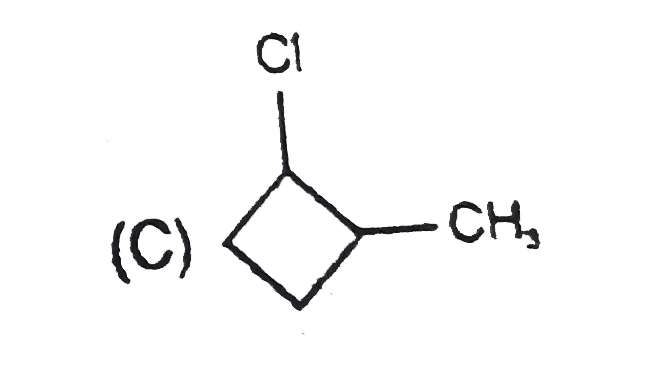
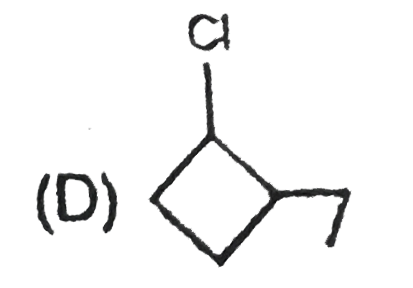
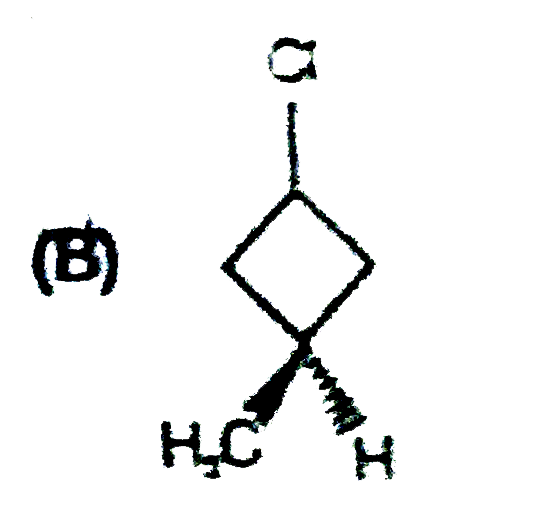A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- which of the following alcohol is least soluble in water ?
Text Solution
|
- Read the following road map carefully
Text Solution
|
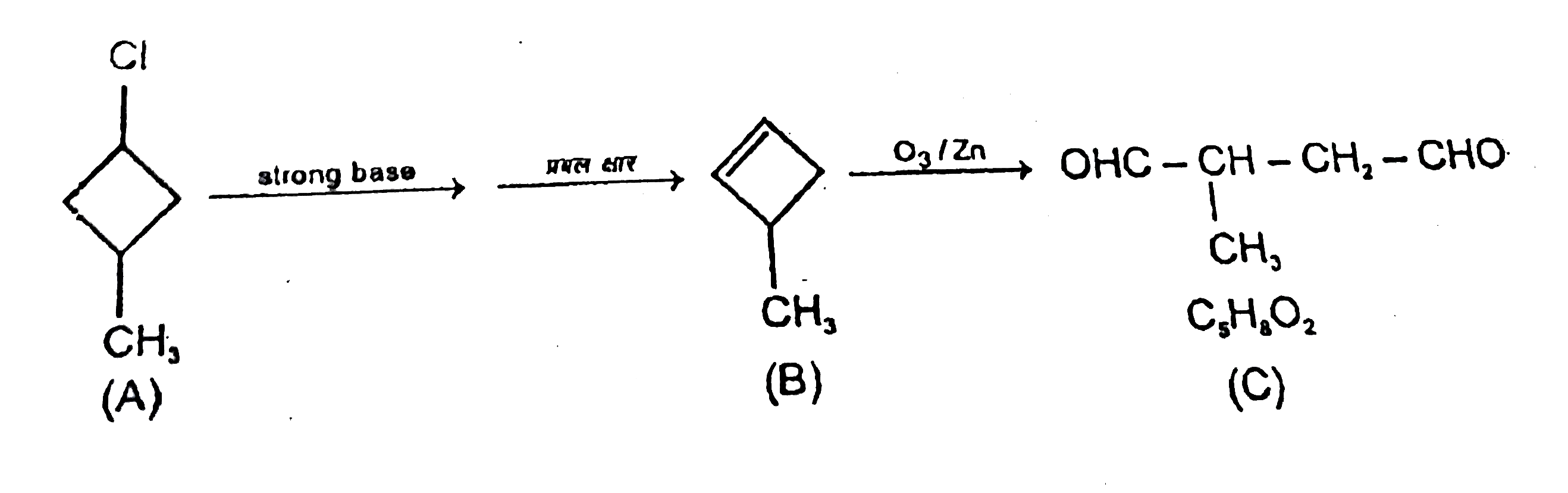
- A compound (A) has molecular formula C(5)H(9)Cl .It does not re...
Text Solution
|
- PhOHunderset(Me2SO4)overset( 2 mol NaOH ) to P In given reaction pr...
Text Solution
|
- Which of the following statement is correct
Text Solution
|
- S(N)1 reaction undergoes through a carbocation intermediate as follows...
Text Solution
|
- Complete the following reaction
Text Solution
|
- CH3-CH2SHunderset((ii)"ethylene oxide")overset((i)CH3O^(ᶱ))toProduct, ...
Text Solution
|
- What is the mole fraction of glucose in 25% w/W glucose solution ?
Text Solution
|
- The mass of NaCl required to prepare 0.5 m aqueous solution in 1kg wat...
Text Solution
|
- The mass of NaCl required to prepare 0.6 m aqueous solution in 1kg wat...
Text Solution
|
- Complete the following reaction
Text Solution
|
- The product of acid hydrolysis of is
Text Solution
|
Text Solution
|
- Neopentyl iodide is treated with aq. AgNO3 solution, a yellow precipit...
Text Solution
|
- The major end product of the following reaction is
Text Solution
|
- Find the mole fraction of solute in its 4 molal aqueous solution.
Text Solution
|
- The correct order of SN 2//E2 ratio for the % yield of product of the ...
Text Solution
|
- What is the mole fraction of glucose in 20% w/W glucose solution ?
Text Solution
|
- overset("Reaction")to Rearranged most stable Carbocation is
Text Solution
|