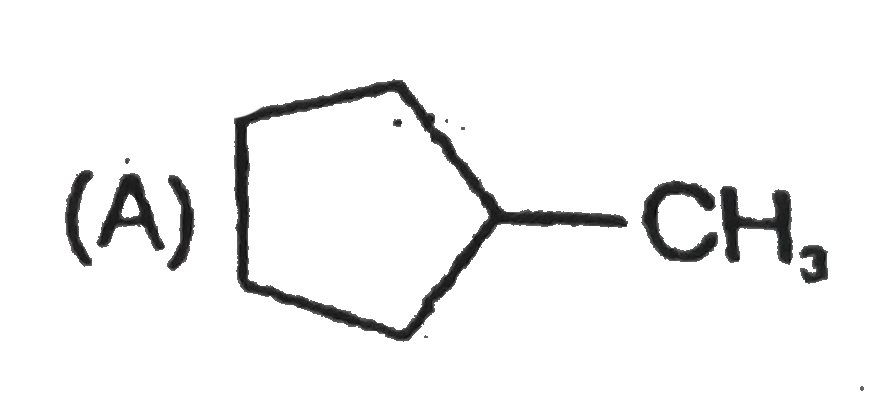
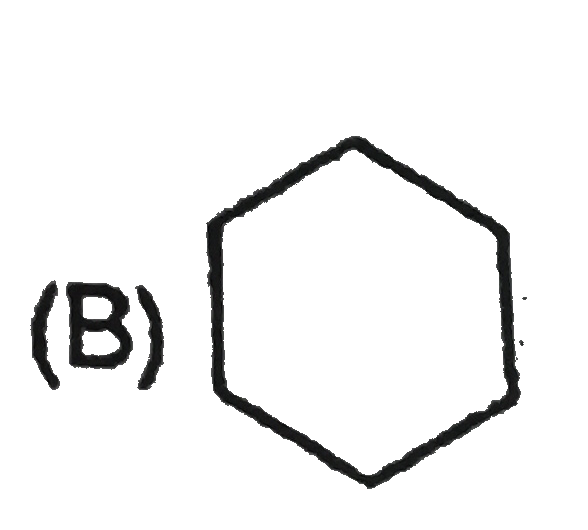
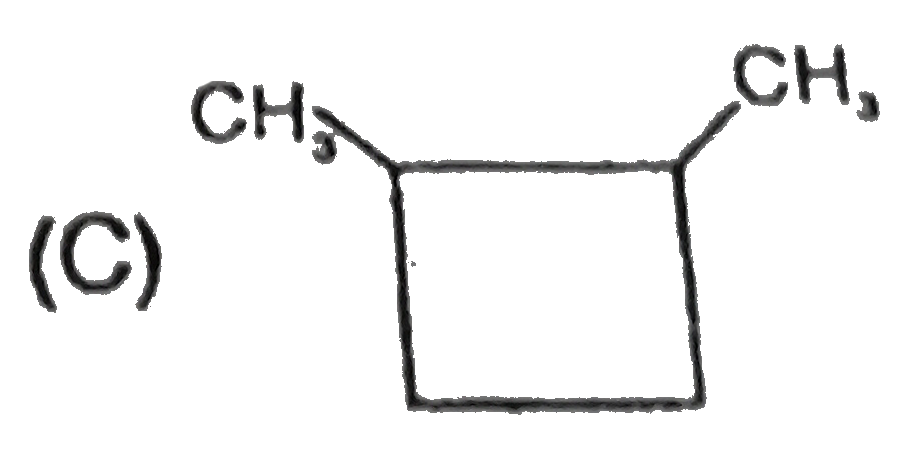
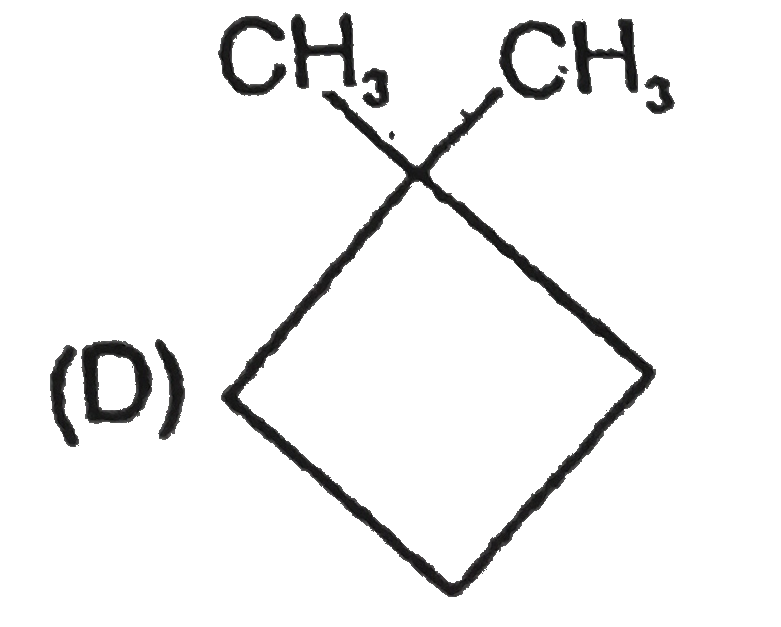
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- A hydrocarbon (X) of the formula C6H12 does not react with bromine wa...
Text Solution
|
- A hydrocarbon (X) of the formula C6H12 does not react with bromine wa...
Text Solution
|
- A hydrocarbon (X) of the formula C6H12 does not react with bromine wa...
Text Solution
|
- A partially racemised (+2) 2-Bromooctane on reaction with aq. NaOH in ...
Text Solution
|
- A partially racemised (+) - 2-bromo-octane (2^@ RX) on reaction with a...
Text Solution
|
- Find the mole fraction of solute in its 7 molal aqueous solution.
Text Solution
|
- Find the mole fraction of solute in its 8 molal aqueous solution.
Text Solution
|
- Find the mole fraction of solute in its 9 molal aqueous solution.
Text Solution
|
- Calculate the weight of lime (CaO) obtained by heating 240kg of 95% pu...
Text Solution
|
- Ether and alcohol are isomers.
Text Solution
|
- In the given reaction how many products are expected.
Text Solution
|
- What is the mole fraction of glucose in 50% w/W glucose solution ?
Text Solution
|
- Find the mole fraction of solute in its 10 molal aqueous solution.
Text Solution
|
- Find the mole fraction of solute in its 11 molal aqueous solution.
Text Solution
|
- Find the mole fraction of solute in its 12 molal aqueous solution.
Text Solution
|
- What is the mole fraction of glucose in 5% w/W glucose solution ?
Text Solution
|
- What is the mole fraction of glucose in 9% w/W glucose solution ?
Text Solution
|
- What is the mole fraction of glucose in 18% w/W glucose solution ?
Text Solution
|
- Find the mole fraction of solute in its 15 molal aqueous solution.
Text Solution
|
- Find the mole fraction of solute in its 14 molal aqueous solution.
Text Solution
|