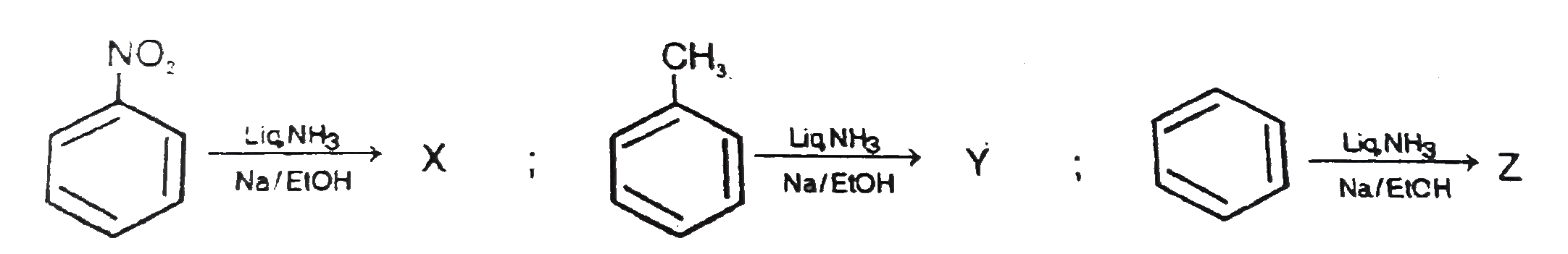
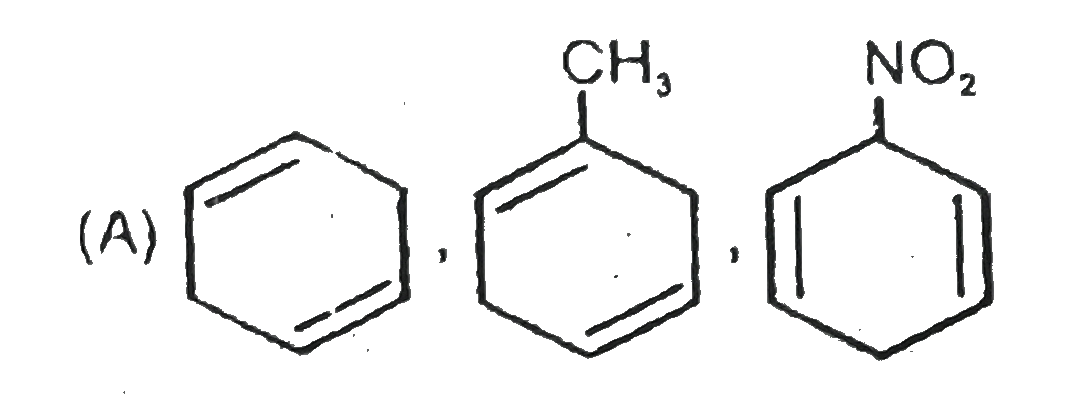
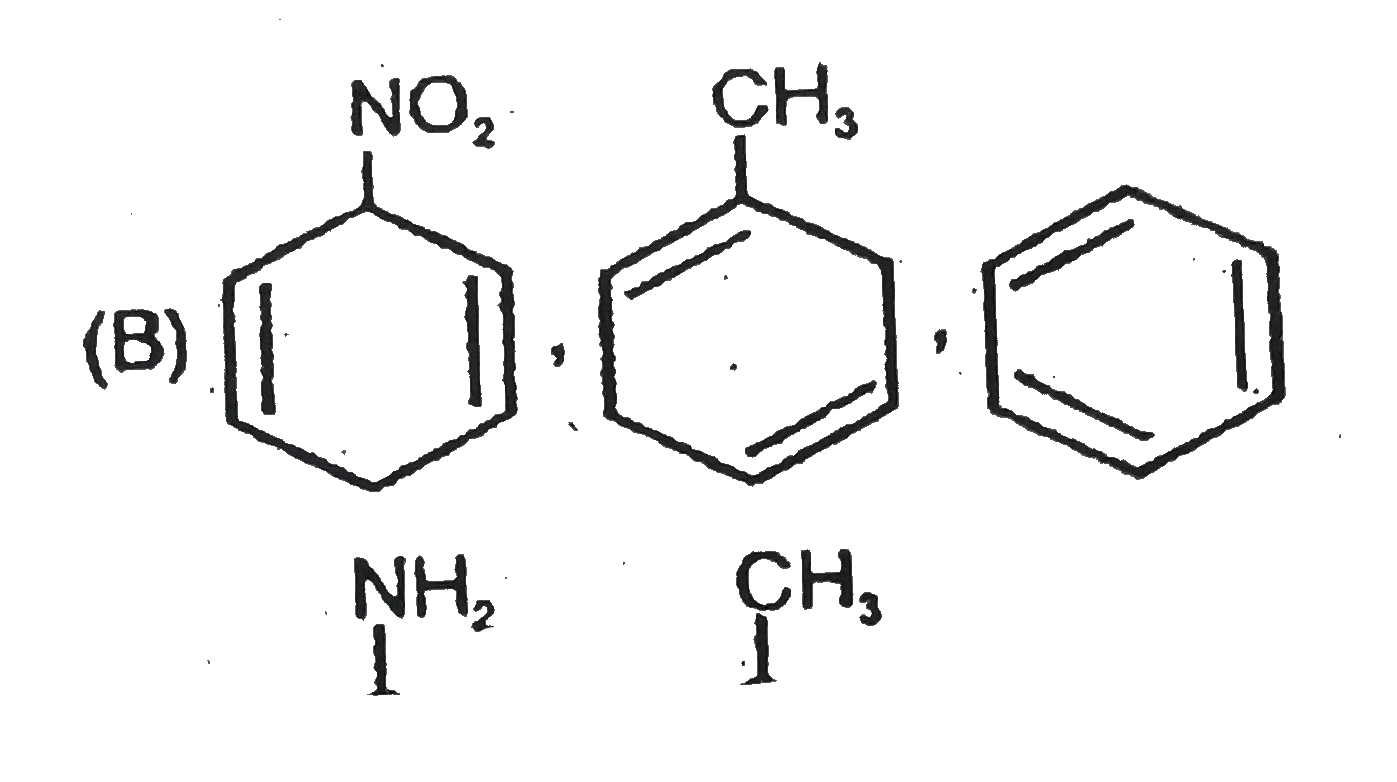
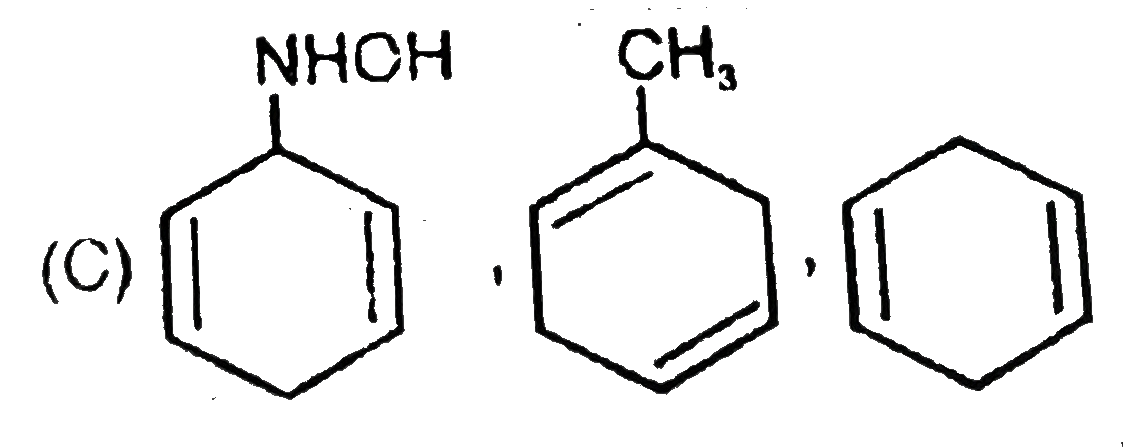
A
B
C
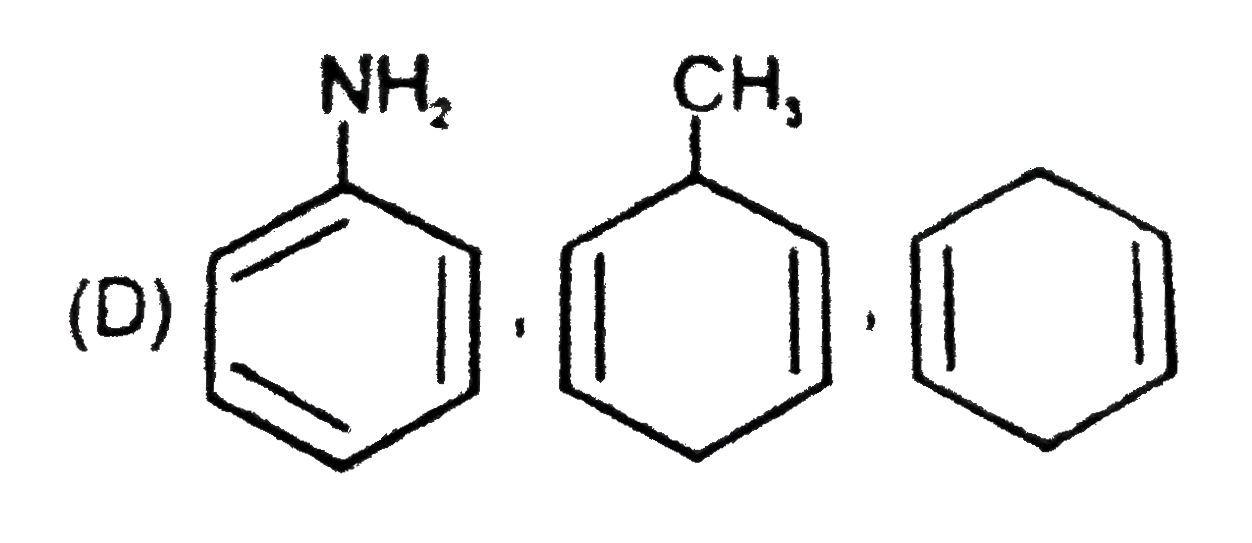
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Find the mole fraction of solute in its 14 molal aqueous solution.
Text Solution
|
- Find the mole fraction of solute in its 13 molal aqueous solution.
Text Solution
|
- The formed products Z,Y,X are respectively .
Text Solution
|
- A hydrocarbon (A) adds one mole of hydrogen in the presence of platinu...
Text Solution
|
- 2-Phenylcycloprop-2-en-1-one is allowed to react with phenylmagnesium ...
Text Solution
|
- 110g of salt is present in 550g of solution. Calculate the mass percen...
Text Solution
|
- The molality of the solution containing 45g of glucose dissolved in 2k...
Text Solution
|
- The end product of following reaction is
Text Solution
|
- The number of atoms in 7.4 moles of Fe are?
Text Solution
|
- Product of the reaction is
Text Solution
|
- CH3-CH2-CH3underset((1))overset(Br2//hv)to(X)underset((2))overset(Mg//...
Text Solution
|
- Ph-N=C=Ounderset((ii)H2O)overset((i)CH3MgBr)toPoverset(LiAH4)toQ P an...
Text Solution
|
- Which of the following reduction methods is not suitable for prepari...
Text Solution
|
- 120g of salt is present in 600g of solution. Calculate the mass percen...
Text Solution
|
- State one method by which carbon dioxide is added to the air.
Text Solution
|
- No . Of products and No . Of fractions are respectively
Text Solution
|
- 90g of salt is present in 450g of solution. Calculate the mass percent...
Text Solution
|
- A sp3 hybrid orbital contains: what percent of S character
Text Solution
|
- The product is
Text Solution
|
- The molality of the solution containing 45g of glucose dissolved in 1k...
Text Solution
|