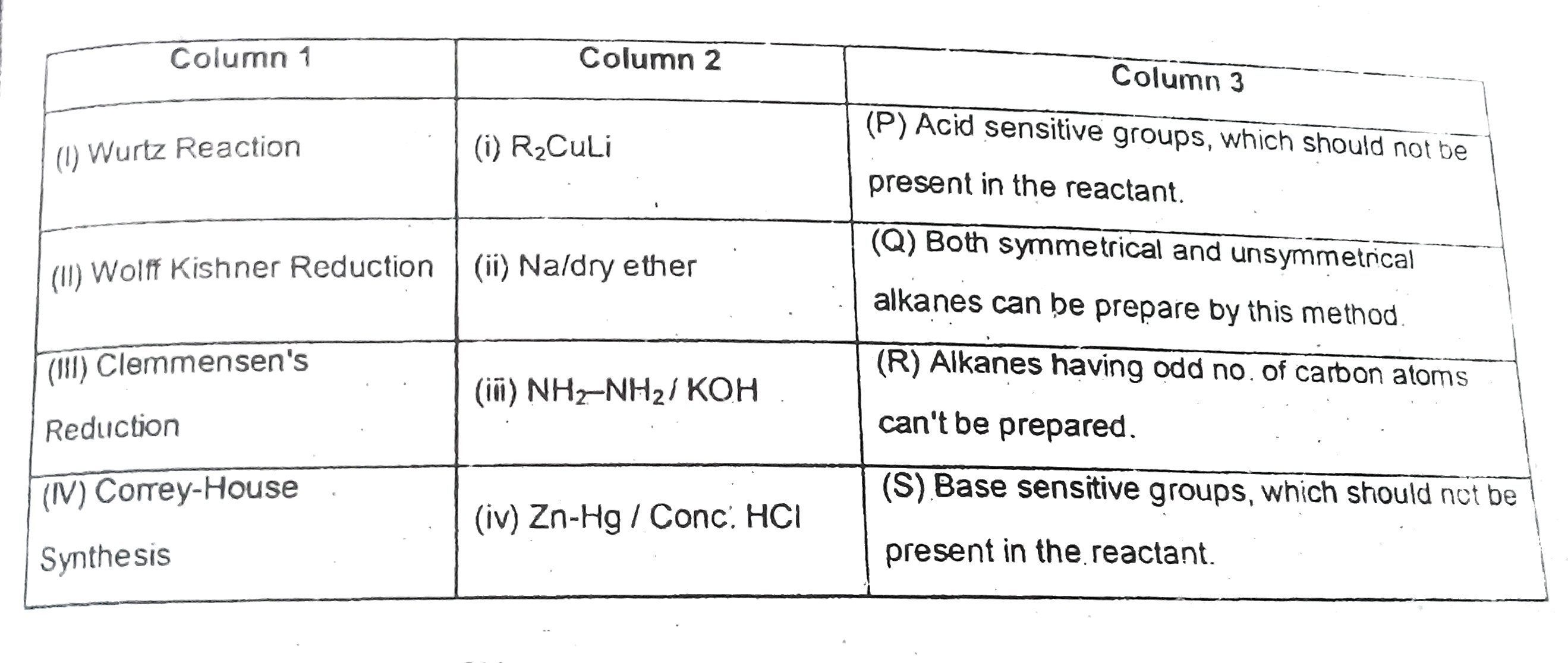
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Observe the following experiment
Text Solution
|
- 15g of salt is present in 75g of solution. Calculate the mass percenta...
Text Solution
|
- 2CH3XtoCH3-CH3 What is the correct combination for the reaction:
Text Solution
|
- What is the correct combination for the reaction :
Text Solution
|
- (X-halogen) What is the correct combination for the reaction :
Text Solution
|
- 20g of salt is present in 100g of solution. Calculate the mass percent...
Text Solution
|
- 40g of salt is present in 200g of solution. Calculate the mass percent...
Text Solution
|
- The molality of the solution containing 65g of glucose dissolved in 1k...
Text Solution
|
- In the following series of reactions the major products P and S are r...
Text Solution
|
- The molality of the solution containing 70g of glucose dissolved in 1k...
Text Solution
|
- The major product of the following product of the following reaction...
Text Solution
|
- The molality of the solution containing 70g of glucose dissolved in 2k...
Text Solution
|
- The number of atoms in 2.4 moles of Fe are?
Text Solution
|
- The number of atoms in 11.5 moles of Fe are?
Text Solution
|
- The end product 'W' in the following sequence of reactions is :
Text Solution
|
- The number of atoms in 10.6 moles of Fe are?
Text Solution
|
- Ph-C-=C-Ph overset(Na//NH3)toAoverset(HC Cl//H^+)toB The product B is
Text Solution
|
- The most stable conformation of the product of following reaction
Text Solution
|
- In the given reaction ,
Text Solution
|
- Which of the following compounds would liberate two moles of methane w...
Text Solution
|