A
B
C
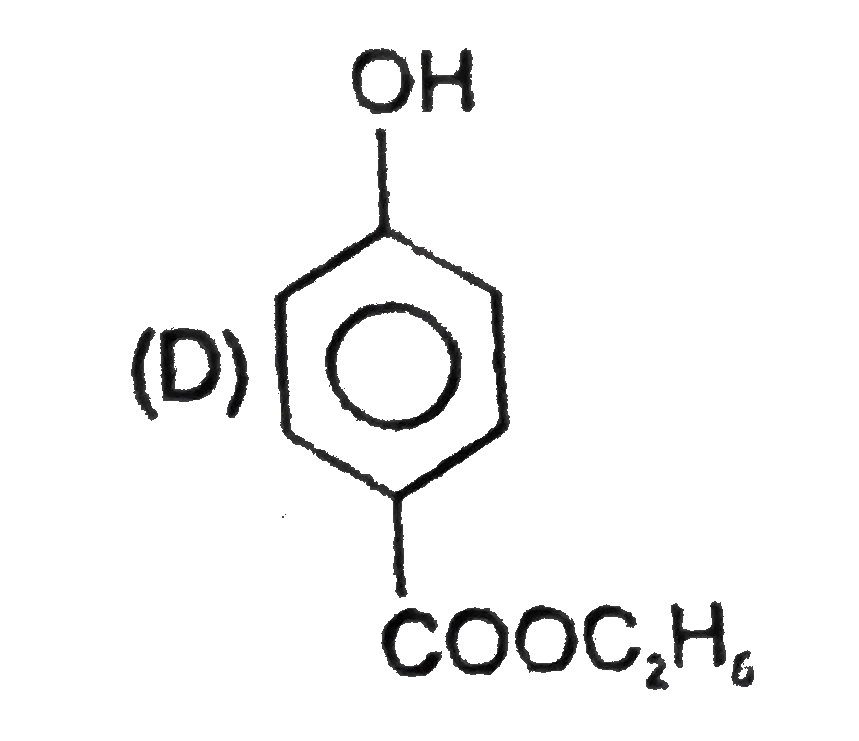
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- The most stable conformation of the product of following reaction
Text Solution
|
- In the given reaction ,
Text Solution
|
- Which of the following compounds would liberate two moles of methane w...
Text Solution
|
- The molality of the solution containing 75g of glucose dissolved in 3k...
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- The number of atoms in 6.5mole of Na are?
Text Solution
|
- Which of the following will be the correct product (P) for the given r...
Text Solution
|
- Xunderset((ii)H3O^(o+))overset((i)KMnO4//H2O // Delta)toHOOC-oversetov...
Text Solution
|
- The end product is
Text Solution
|
- The final product of the following reaction is
Text Solution
|
- Identify structure of compound (D).
Text Solution
|
- When But-2-yne is treated with H(2) / Pd-BaSO(4) the product formed w...
Text Solution
|
- Product obtained after First step of above mentioned reaction is:
Text Solution
|
- The following synthesis can not be carried out by :
Text Solution
|
- Bu-C=CHoverset(LiNH2)toAunderset((ii)H2O)overset((i)PhCHO)toBoverset(M...
Text Solution
|
- Which of the following methods yield saturated hydrocarbon ?
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- The number of atoms in 2.5mole of Na are?
Text Solution
|
- Statement-1:Reduction of But-2-yne of Na/liq. NH3 gives trans-But-2-...
Text Solution
|
- When HBr adds to 1-butene in the presence of benzoyl peroxide, the pro...
Text Solution
|