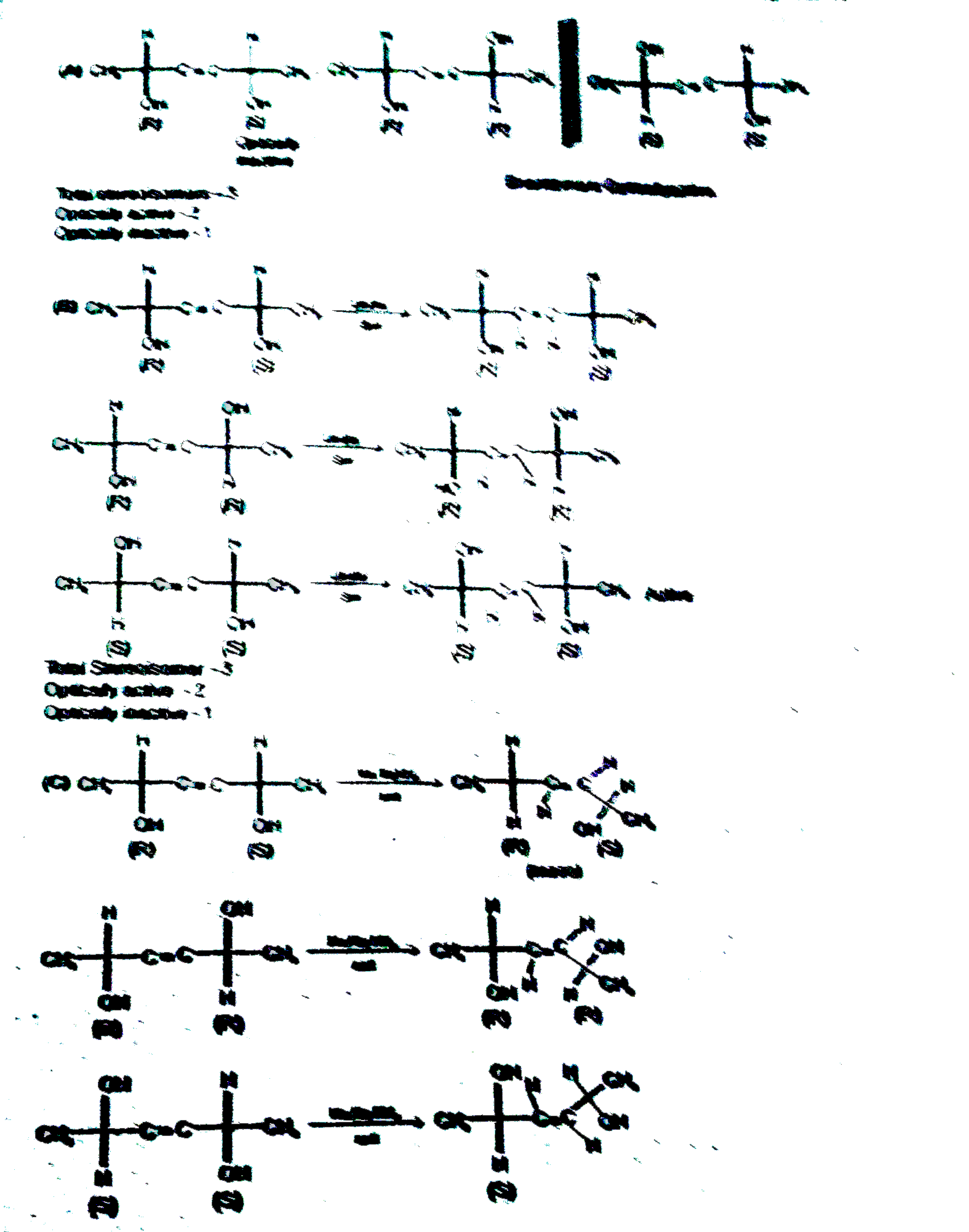
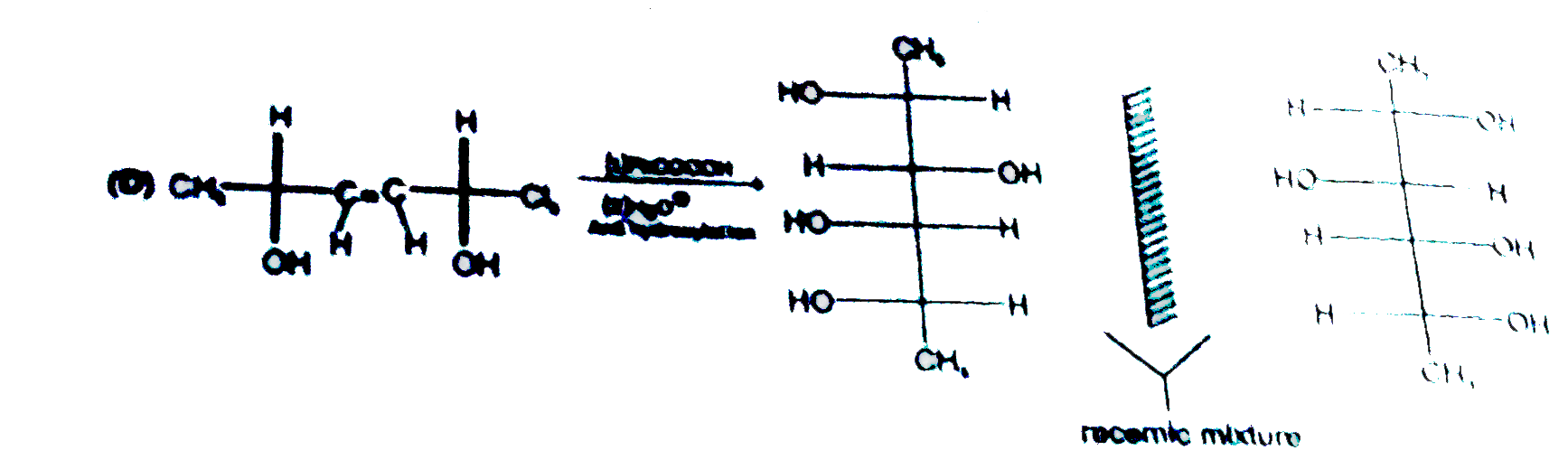
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- CH3-CH=CH2 reacts with Cl2 at 500^@C Find out total no . Of possible...
Text Solution
|
- The number of atoms in 7.4mole of Na are?
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- The number of atoms in 0.4mole of Na are?
Text Solution
|
- The number of atoms in 0.6mole of Na are?
Text Solution
|
- When nucleophile encounters a ketone site of attack is
Text Solution
|
- The only correct combination that reaction nature is stereospecific an...
Text Solution
|
- When primary amine reacts with chloroform in ethanolic KOH then produc...
Text Solution
|
- Study the following reactions and identify the reactant 'R'. It can be
Text Solution
|
- Which can be the product of the following reaction
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- The major product is
Text Solution
|
- The product is
Text Solution
|
- Ph-underset(Me)underset(|)overset(OH)overset(|)(C)-underset(I)underset...
Text Solution
|
Text Solution
|
- Why does the reaction produce stable salt ? Because
Text Solution
|
- The major product of reaction is :
Text Solution
|