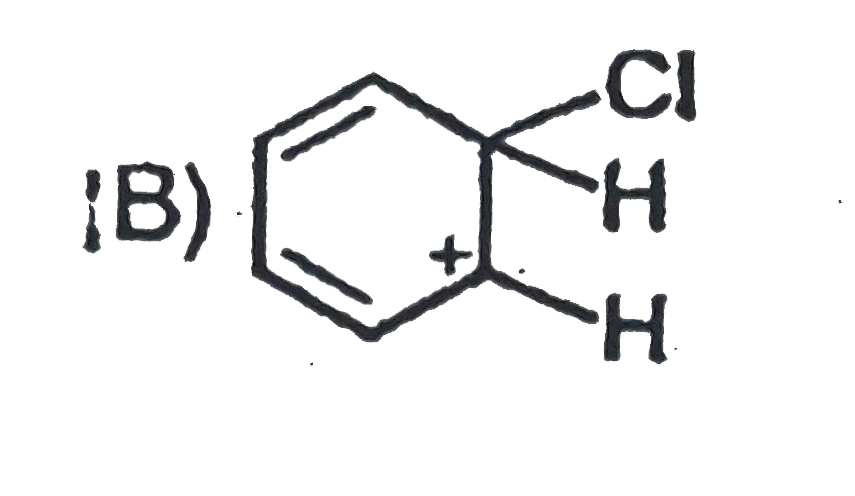
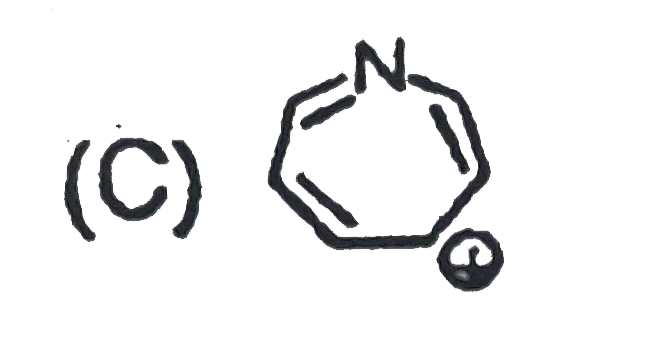
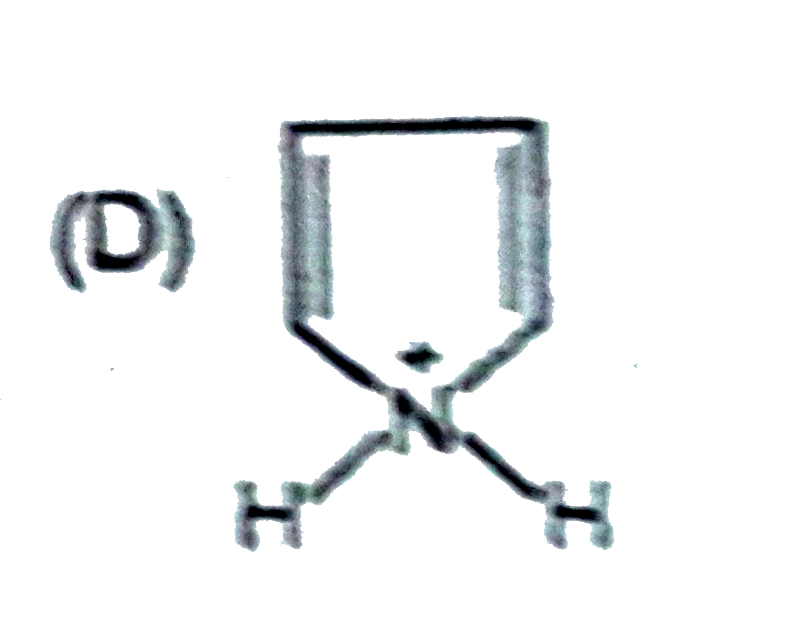
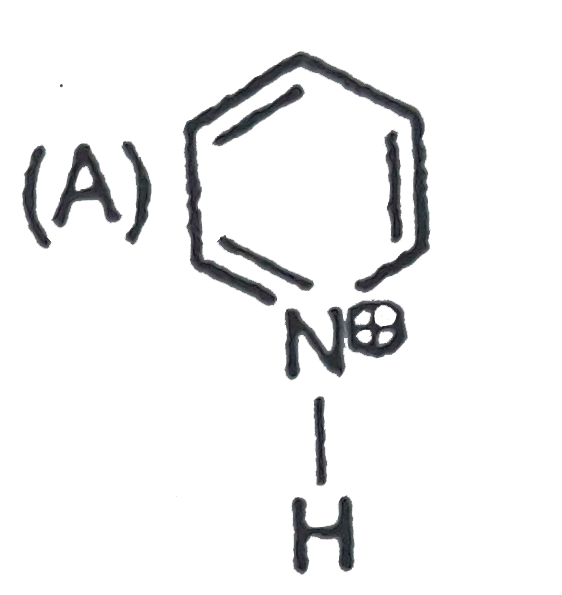A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Which of the following reactions give alkylation product :
Text Solution
|
- The following conversion reaction can be carried out by using reacti...
Text Solution
|
- Which of the folllowing ion will be aromatic in nature ?
Text Solution
|
- Statement-1:Benzene and ethene both give reactions with electrophillic...
Text Solution
|
- Statement-1: Statement-2:Coplanar arrangement of three rings is flu...
Text Solution
|
- Statement-1:Polyacylation in benzene does not occur during Friedal Cra...
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- The general electrophilic substitution reaction (replacement of H. by ...
Text Solution
|
- The general electrophilic substitution reaction (replacement of H. by ...
Text Solution
|
- The product U is
Text Solution
|
- Which of the following will not be the product when S on reaction with...
Text Solution
|
- The compound C8H9Cl(A) on treatments with KCN followed by hydrolysis g...
Text Solution
|
- The compound C8H9Cl(A) on treatments with KCN followed by hydrolysis g...
Text Solution
|
- The compound C8H9Cl(A) on treatments with KCN followed by hydrolysis g...
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- Hydration of 3-phenylbut-1-ene in dil H(2)So(4) will give mainly :
Text Solution
|
- The number of atoms in 0.3mole of Na are?
Text Solution
|
- The number of atoms in 0.7mole of Na are?
Text Solution
|