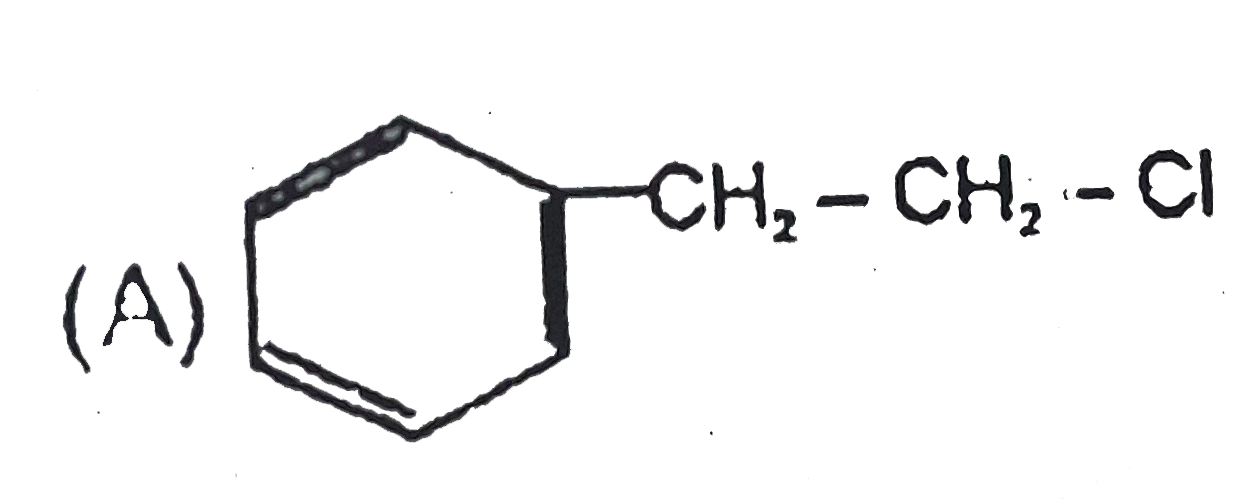
A
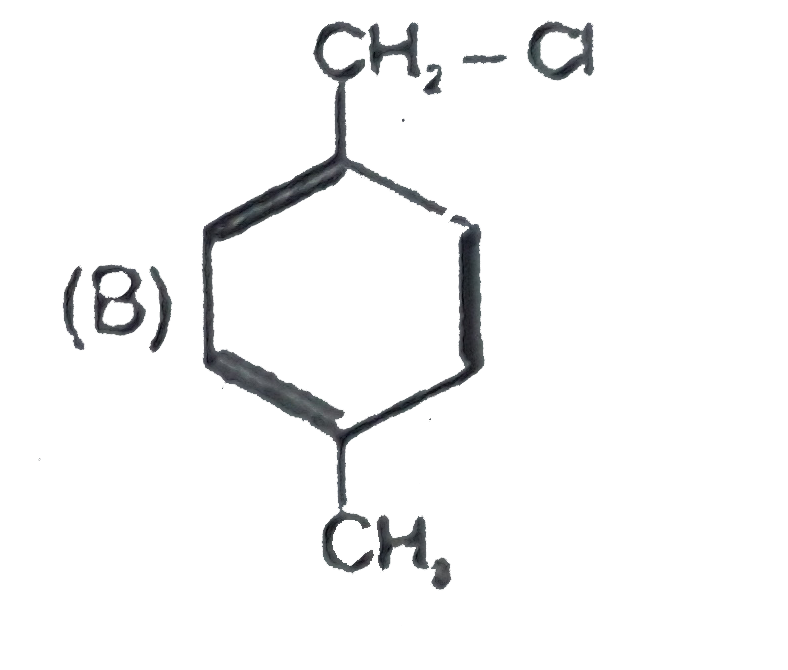
B
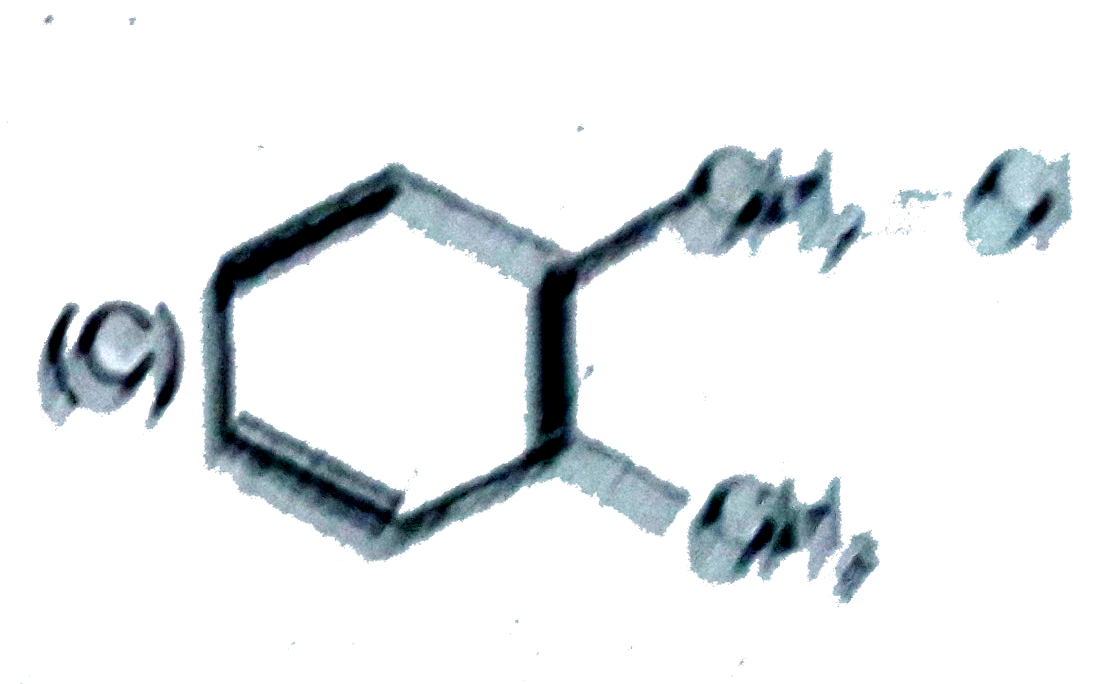
C
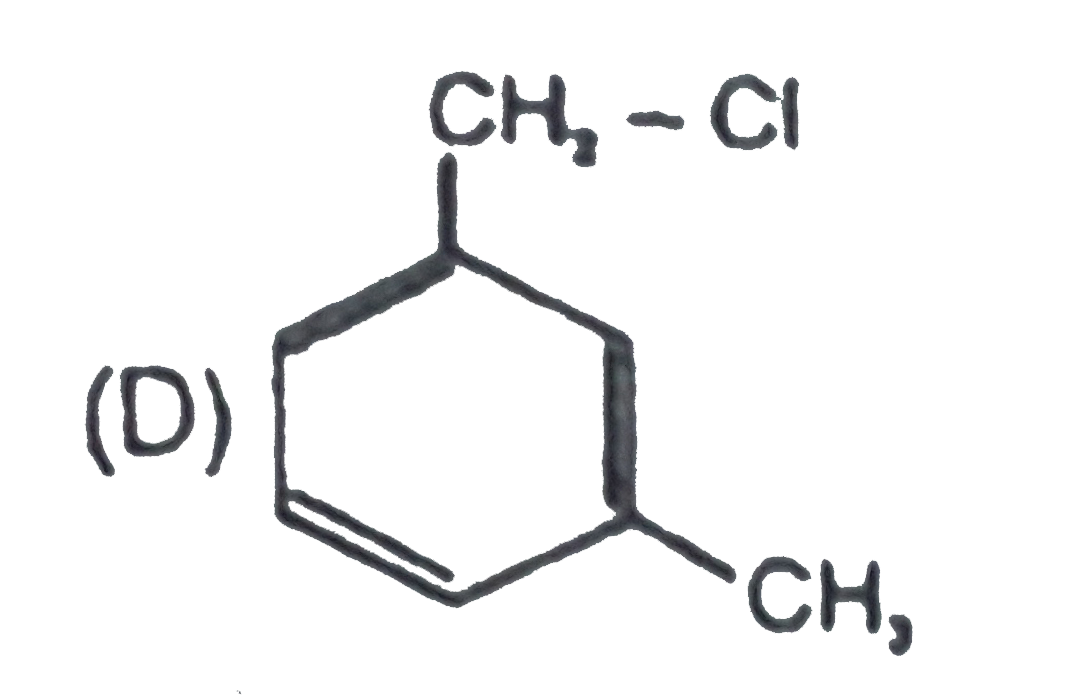
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- The product U is
Text Solution
|
- Which of the following will not be the product when S on reaction with...
Text Solution
|
- The compound C8H9Cl(A) on treatments with KCN followed by hydrolysis g...
Text Solution
|
- The compound C8H9Cl(A) on treatments with KCN followed by hydrolysis g...
Text Solution
|
- The compound C8H9Cl(A) on treatments with KCN followed by hydrolysis g...
Text Solution
|
- Mole fraction of ethyl alcohol in aqueous ethyl alcohol (C2H5OH) solut...
Text Solution
|
- Hydration of 3-phenylbut-1-ene in dil H(2)So(4) will give mainly :
Text Solution
|
- The number of atoms in 0.3mole of Na are?
Text Solution
|
- The number of atoms in 0.7mole of Na are?
Text Solution
|
- The number of atoms in 0.8mole of Na are?
Text Solution
|
- Match the correct properties of compounds of column I with column II.
Text Solution
|
- The steps involved in the above reaction can be arranged can be arrang...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 50 per cent ...
Text Solution
|
- Find the structure formula and molecular weight of 'P'.
Text Solution
|
- Indicates the most probable sites for ArSE reactions.
Text Solution
|
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- The only correct combination in which Azo dye is formed.
Text Solution
|
- The only correct combination in which isocyanide is formed
Text Solution
|
- Choose correct combination for x,y and z
Text Solution
|