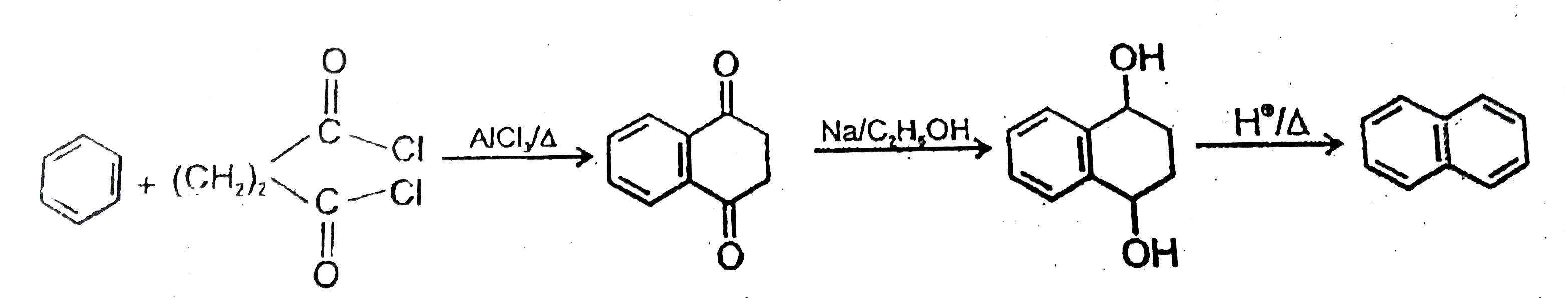
Text Solution
Verified by Experts
The correct Answer is:
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Find the structure formula and molecular weight of 'P'.
Text Solution
|
- Indicates the most probable sites for ArSE reactions.
Text Solution
|
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- The only correct combination in which Azo dye is formed.
Text Solution
|
- The only correct combination in which isocyanide is formed
Text Solution
|
- Choose correct combination for x,y and z
Text Solution
|
- Choose correct combination for x,y and z
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- The compound (U) is (the major isomer is ):
Text Solution
|
- C12H10 is an aromatic compound which on ozonolysis gives mixture of Gl...
Text Solution
|
- If true enter 1, else enter 0. sp^2 hybrid orbitals have equal s ...
Text Solution
|
- Density is highest for :
Text Solution
|
- An unsaturated hydrocarbon on complete hydrogenation gives 1-isopropl-...
Text Solution
|
- Define Action of heat on aluminium hydroxide.
Text Solution
|
- Which test is used to distinguish between Phenols and aliphatic alcoho...
Text Solution
|
- Complete the following reaction
Text Solution
|
- The following two compounds I and II can be distinguished by using rea...
Text Solution
|
- Consider following compounds and decide as to which of the following s...
Text Solution
|
- An aromatic compound 'X' (C9H8O3) turns blue litmus to red.It gives ye...
Text Solution
|