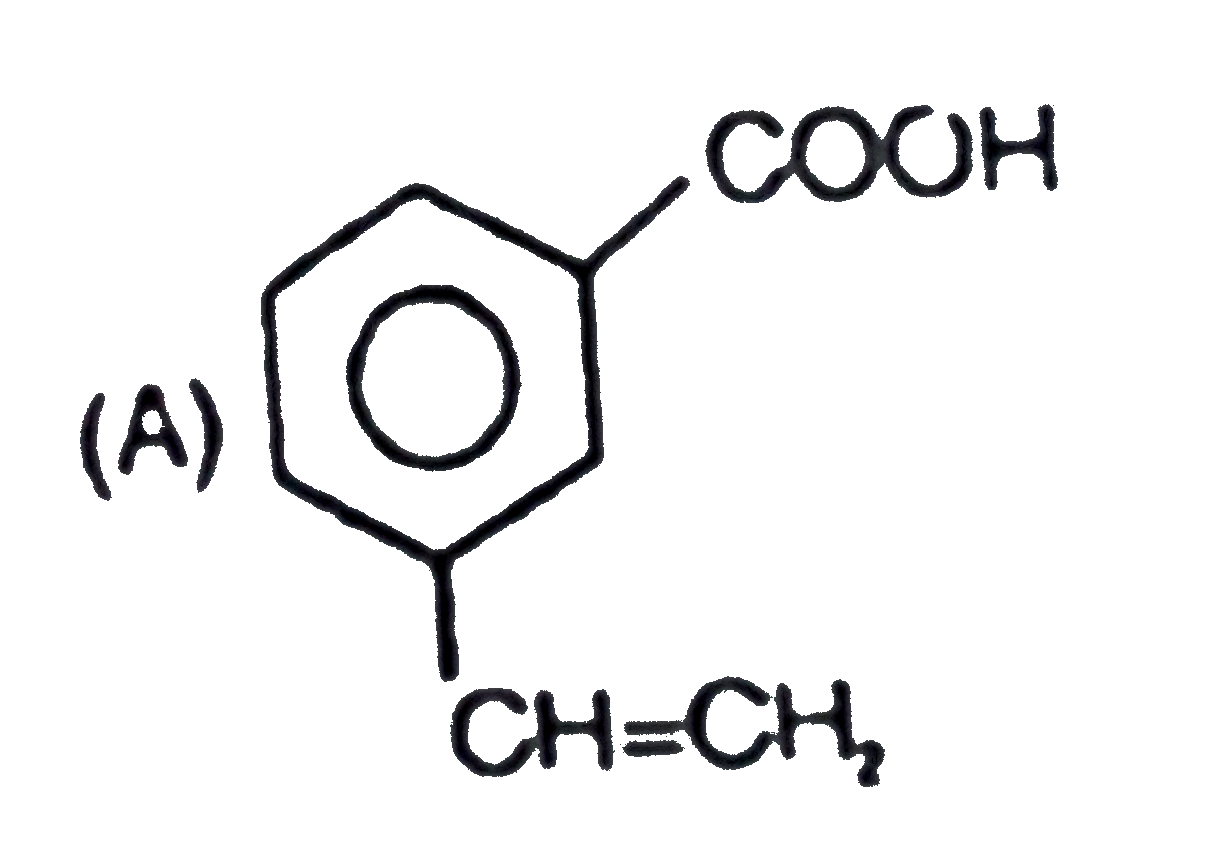
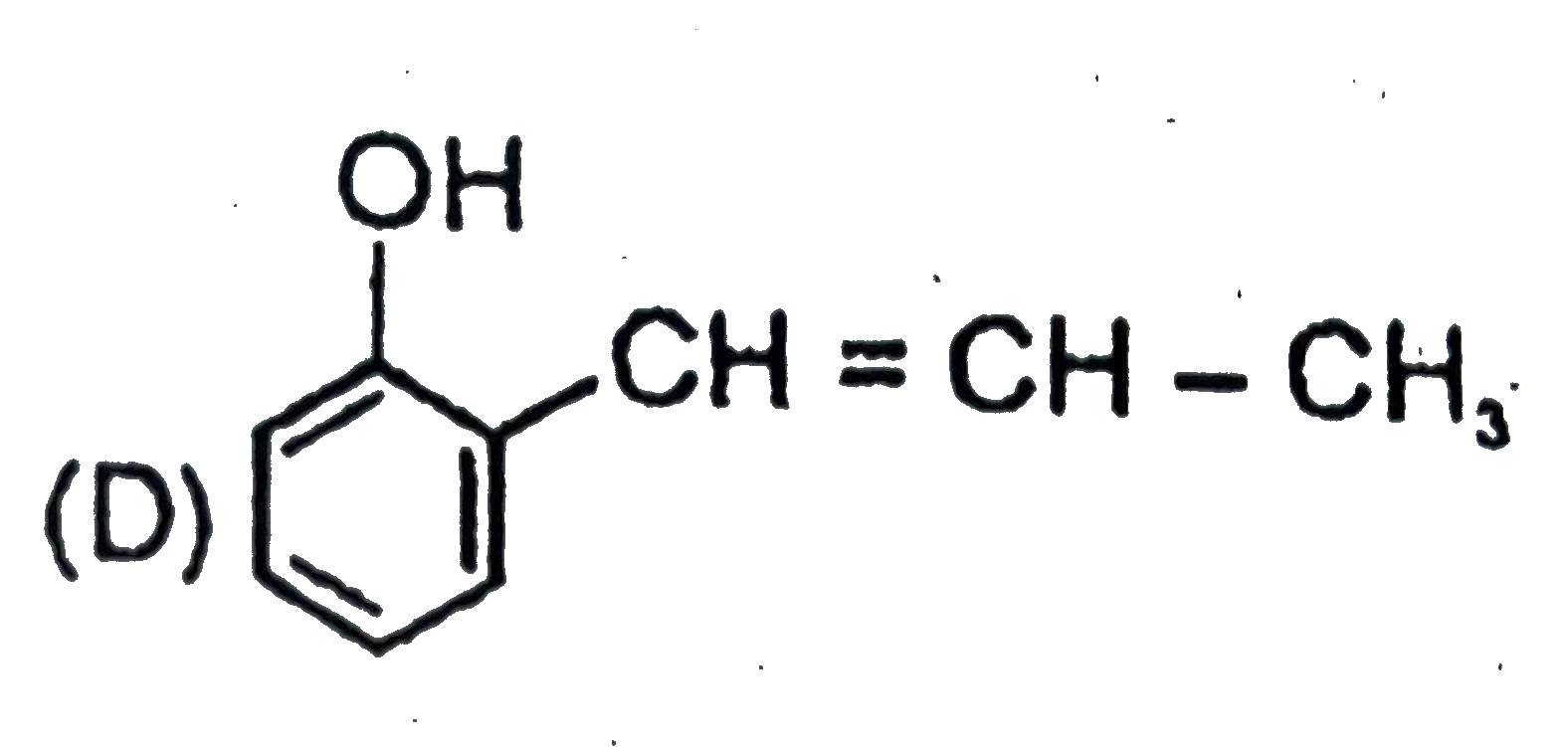
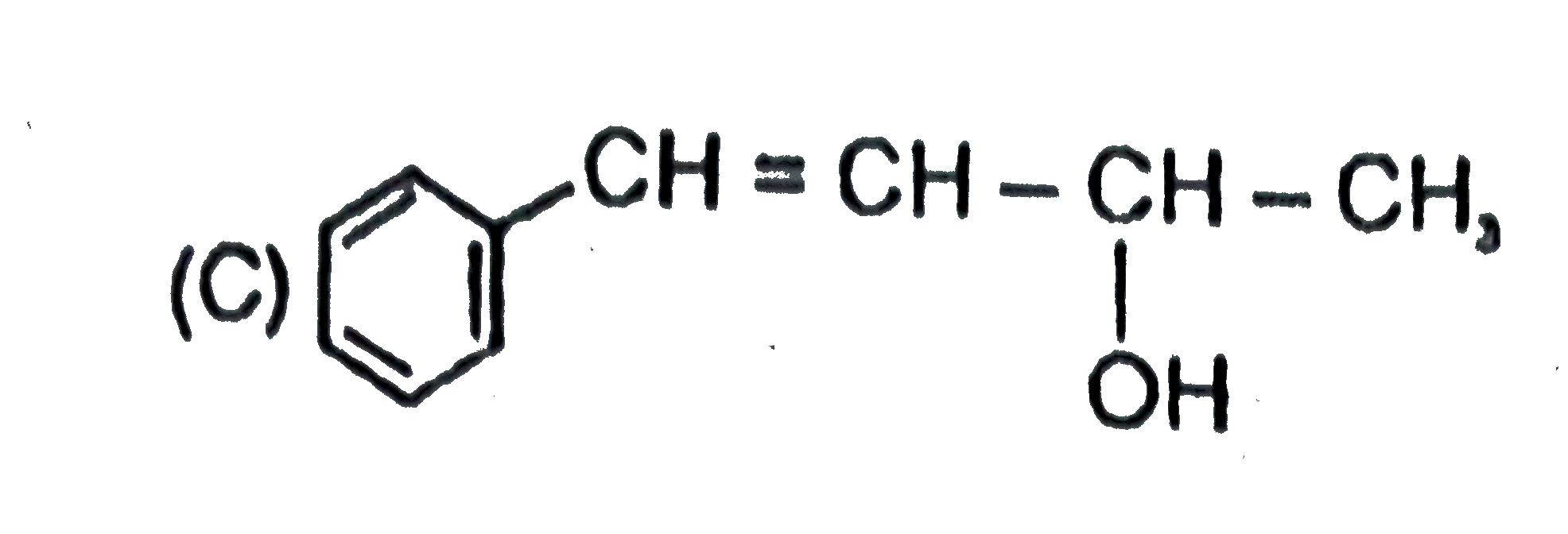A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- 2D,3L,4D,5D-6-Pentahhydroxyhexanal can give.
Text Solution
|
- Compound X and Y both have the same molecular formula (C4H8O).They giv...
Text Solution
|
- Compound 'P' (C10H12O) evolves H2 gas with Na metal.It reacts with Br2...
Text Solution
|
- How will you convert Methyl amine into ethyl amine ?
Text Solution
|
- Statement-1:Aceteldehyde responds positively with all the test of carb...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Observer the following road map of reactions and give answer. The...
Text Solution
|
- Alkene (X) (C(5)H(10)) on ozonolysis gives a mixture of two compounds ...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Which of the following statement is true
Text Solution
|
- Observe the following sequence of reations P shows geometrical is...
Text Solution
|
- Observe the following sequence of reations P shows geometrical is...
Text Solution
|
- Observe the following sequence of reations P shows geometrical is...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 50 per cent ...
Text Solution
|
- Give a chemical test to distinguish between a ethanal and propanal ?
Text Solution
|
- An aromatic compound T (C10H10O2) gives 2 moles of CHI2 and compound U...
Text Solution
|
- Column I may match with more than one conditions of column II. {:("C...
Text Solution
|
- X(C8H14) by ozonolysis forms Y[C8H14O2].Y on reaction with NaOI follow...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|