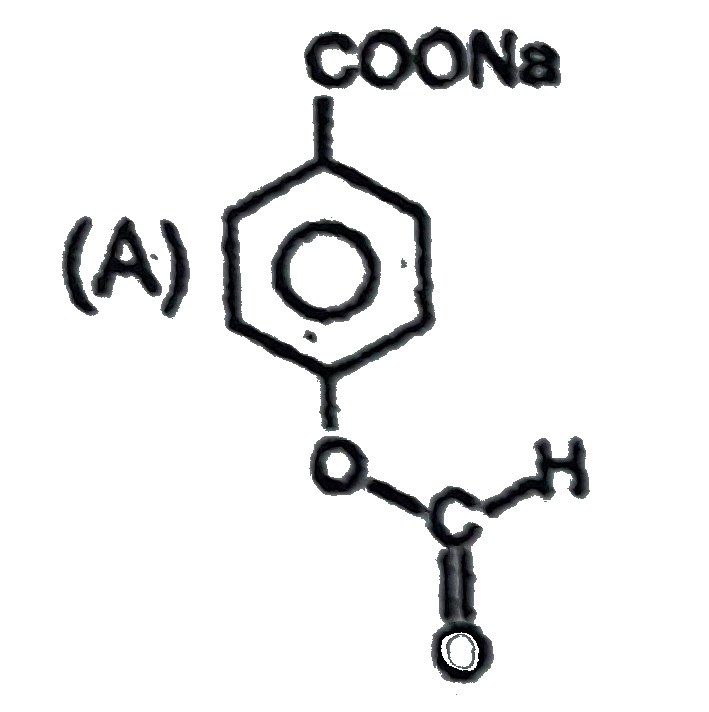
A
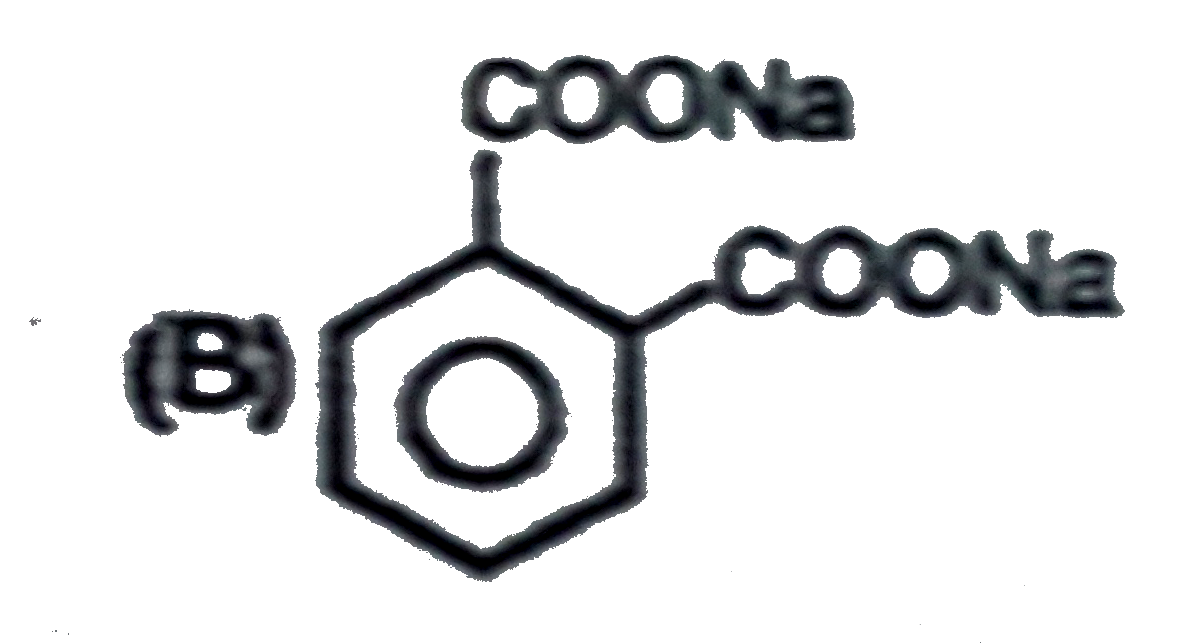
B
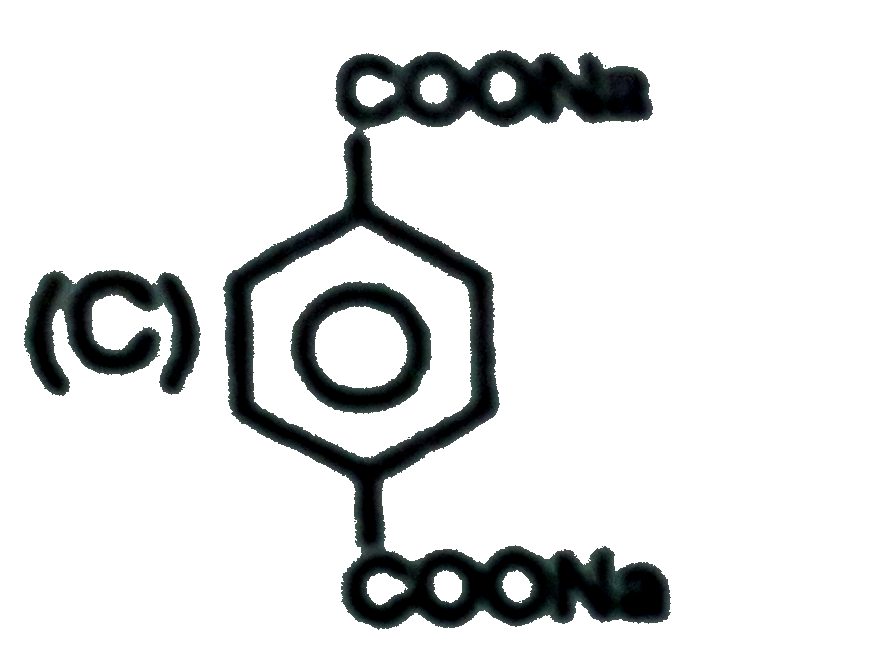
C
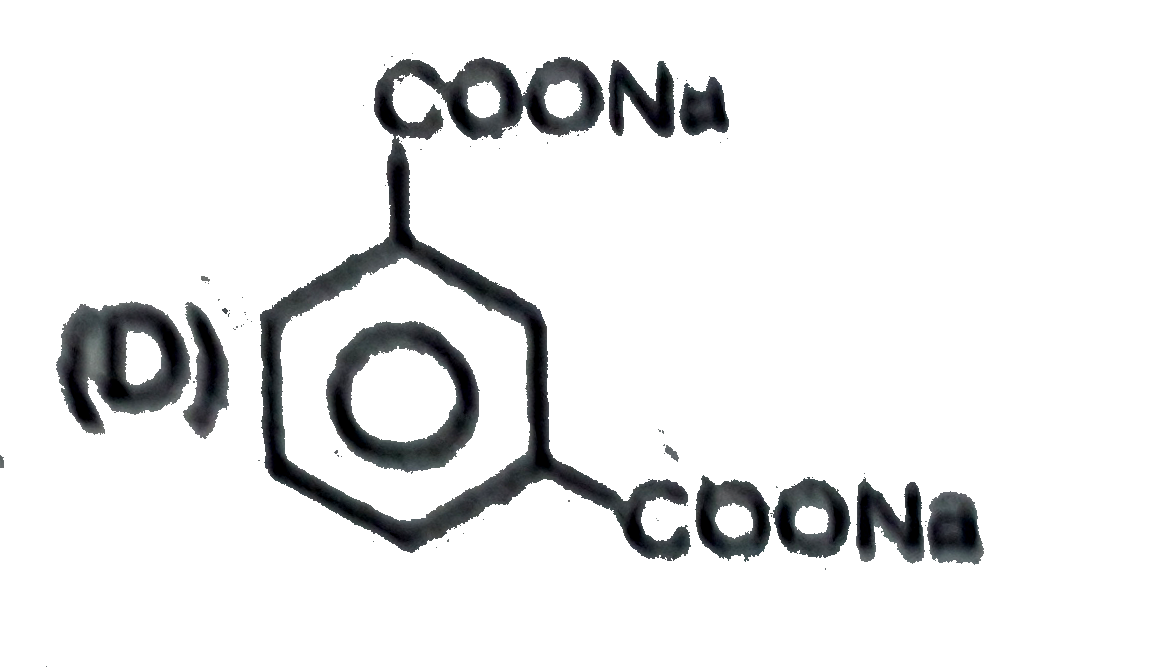
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- How much amount of CaCO3 in gram having percentage purity 50 per cent ...
Text Solution
|
- Give a chemical test to distinguish between a ethanal and propanal ?
Text Solution
|
- An aromatic compound T (C10H10O2) gives 2 moles of CHI2 and compound U...
Text Solution
|
- Column I may match with more than one conditions of column II. {:("C...
Text Solution
|
- X(C8H14) by ozonolysis forms Y[C8H14O2].Y on reaction with NaOI follow...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- How many alkenes can be hydrogenated to give an alkane with molecular ...
Text Solution
|
- How will you prepare acetone from Acetyl chloride ?
Text Solution
|
- Which of the following set gives the product that give two monochloro ...
Text Solution
|
- Which of the following combination gives homocyclic unsaturated produc...
Text Solution
|
- Give the correct set for the product which can be obtained by the hydr...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Which carbonyl group is protonated most readily in acidic solution, bu...
Text Solution
|
- PhCOCHBr(2)overset(OH^(-))toAoverset(OH^(-))toBoverset(H^(+))toC The...
Text Solution
|
- The product of the following reaction is
Text Solution
|
- Which step is not feasible in the following loop ?
Text Solution
|
- How will you prepare Aspirin from Salicylic Acid ? Give two uses of As...
Text Solution
|
- PhCHO+(CH3CO)2Ooverset(CH3COONa)toAoverset(HBr)toB The product B is
Text Solution
|