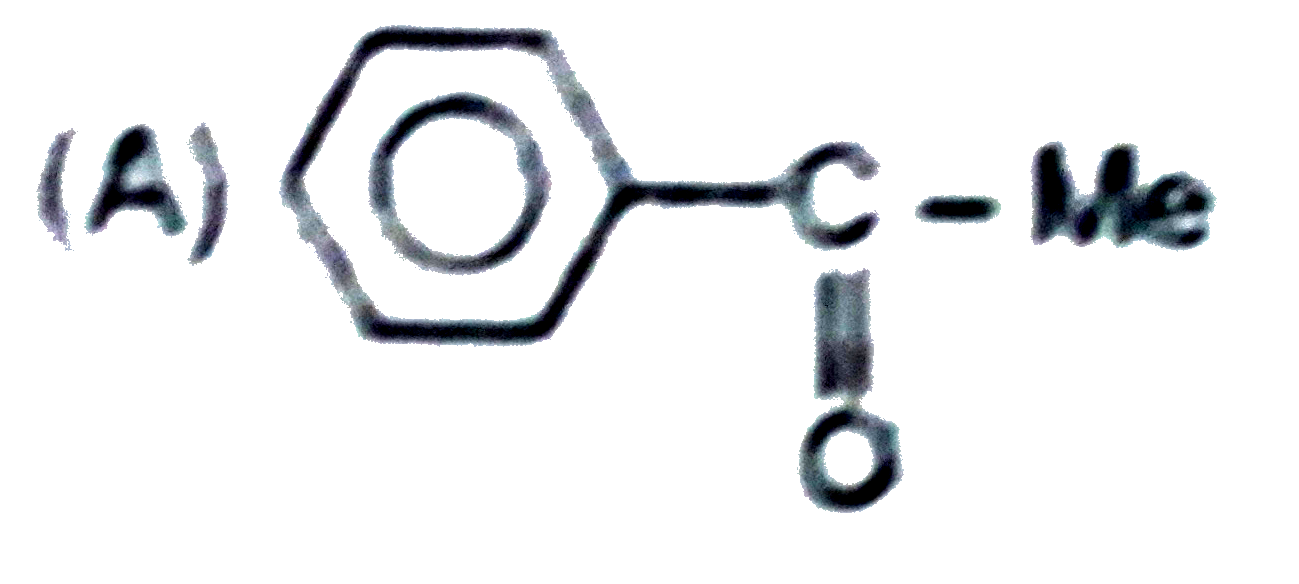
A
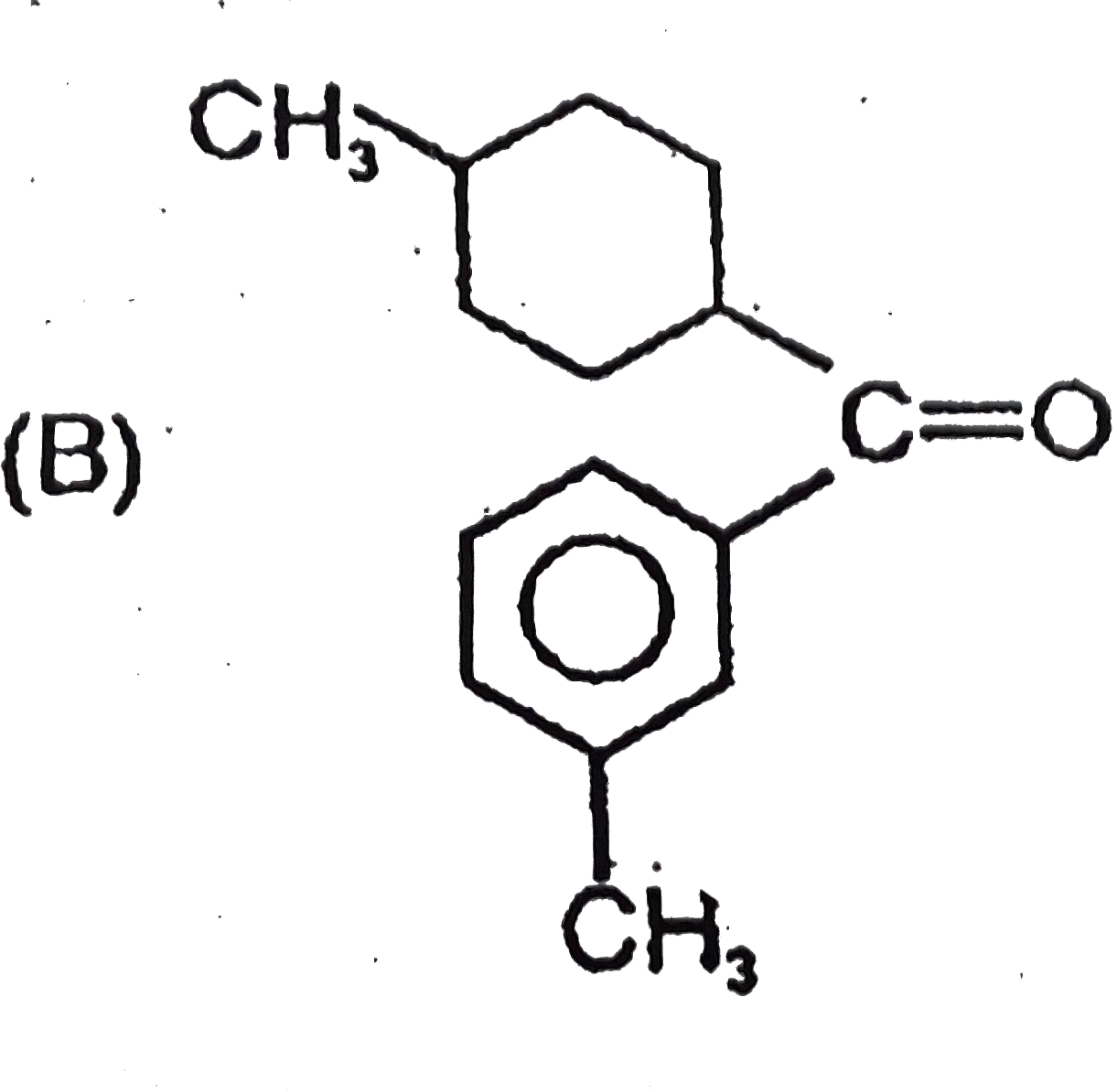
B
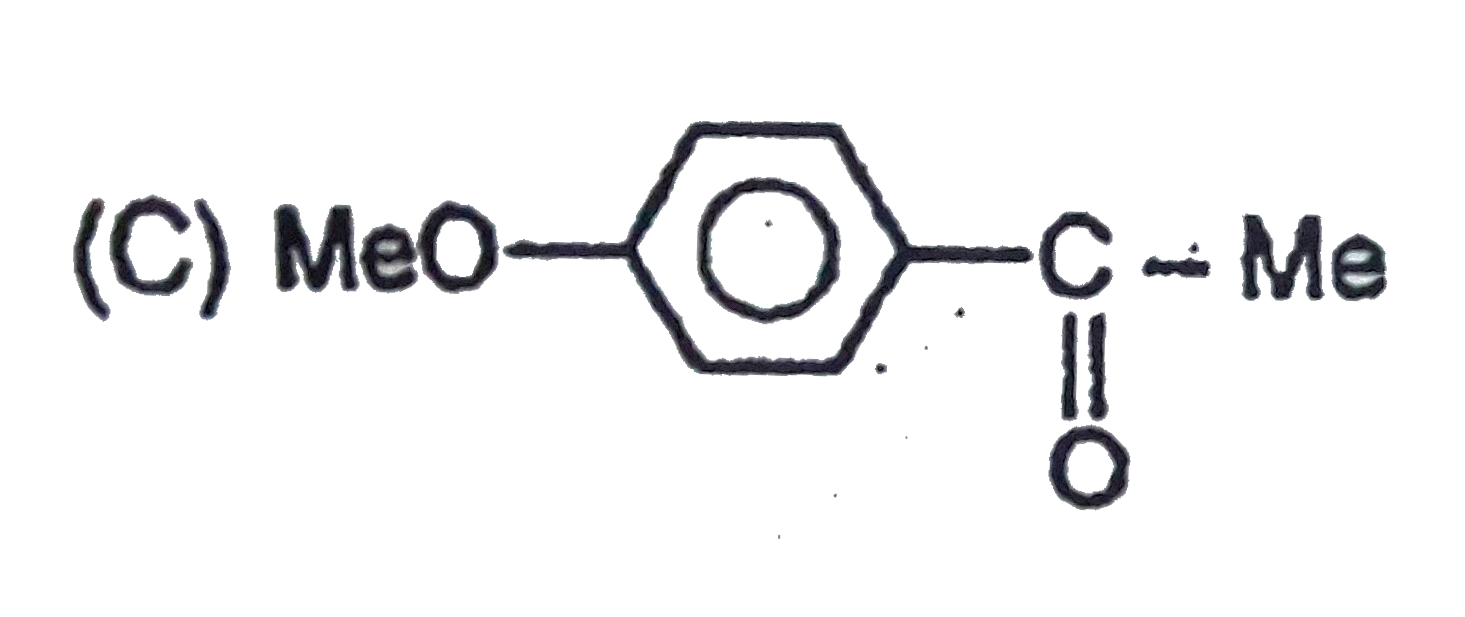
C
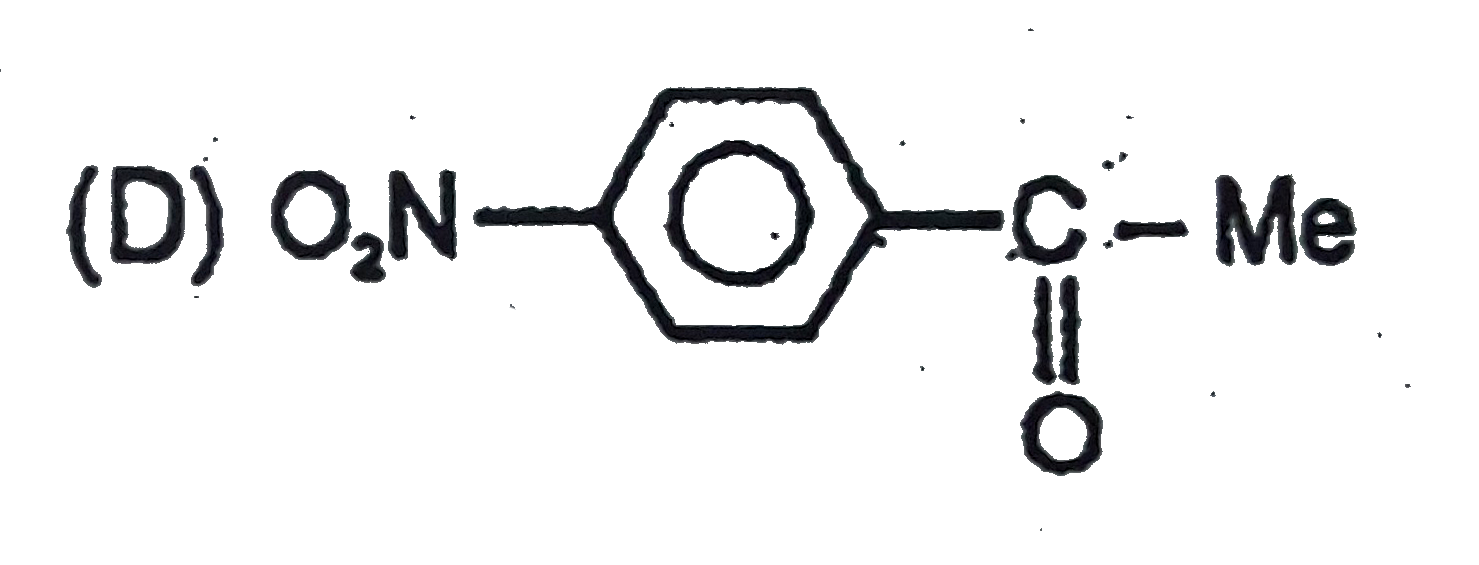
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Give the correct set for the product which can be obtained by the hydr...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Which carbonyl group is protonated most readily in acidic solution, bu...
Text Solution
|
- PhCOCHBr(2)overset(OH^(-))toAoverset(OH^(-))toBoverset(H^(+))toC The...
Text Solution
|
- The product of the following reaction is
Text Solution
|
- Which step is not feasible in the following loop ?
Text Solution
|
- How will you prepare Aspirin from Salicylic Acid ? Give two uses of As...
Text Solution
|
- PhCHO+(CH3CO)2Ooverset(CH3COONa)toAoverset(HBr)toB The product B is
Text Solution
|
- The number of stereoisormeric products fromed in the following reactio...
Text Solution
|
- In which of the following compound O^18 exchange can be observed on ke...
Text Solution
|
- What will be the product of the following reaction
Text Solution
|
- The possible number of stereoisomers of the product of following react...
Text Solution
|
- What is hemiacetal form ? Give an example in each case ?
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Complete the following reaction
Text Solution
|
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 50 per cent ...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 50 per cent ...
Text Solution
|