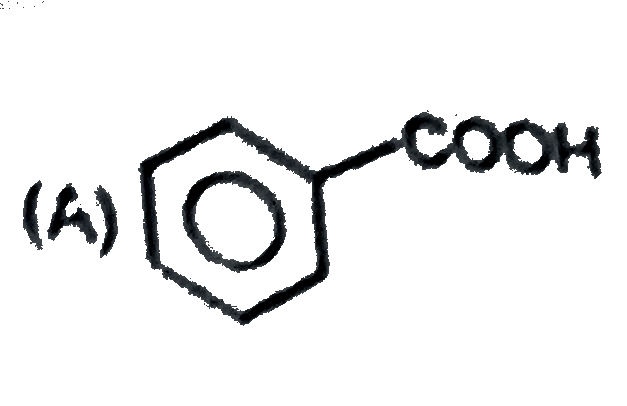
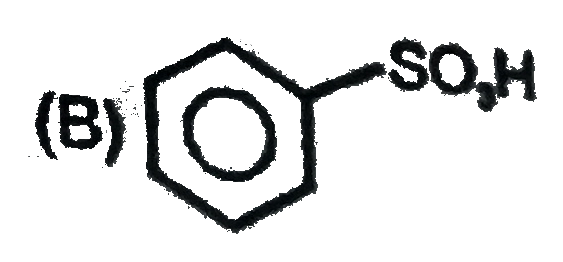
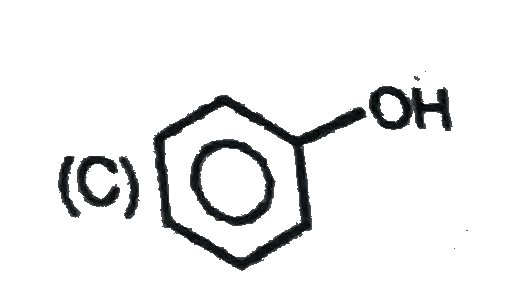
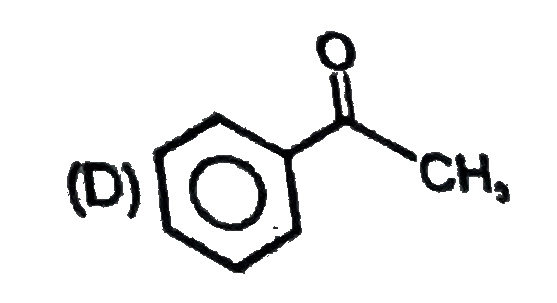
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- PhCHO+(CH3CO)2Ooverset(CH3COONa)toAoverset(HBr)toB The product B is
Text Solution
|
- The number of stereoisormeric products fromed in the following reactio...
Text Solution
|
- In which of the following compound O^18 exchange can be observed on ke...
Text Solution
|
- What will be the product of the following reaction
Text Solution
|
- The possible number of stereoisomers of the product of following react...
Text Solution
|
- What is hemiacetal form ? Give an example in each case ?
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Calculate the mass of iron which will be converted into its oxide (Fe3...
Text Solution
|
- Complete the following reaction
Text Solution
|
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 50 per cent ...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 50 per cent ...
Text Solution
|
- When Benzene-1,3-dicarbaldehyde react with 50% NaOH.The products forme...
Text Solution
|
- Alcohols react with HCL or PCL(5) , but phenol does not Why ?
Text Solution
|
- What is Acetal form ? give example .
Text Solution
|
- Calculate the weight of lime (CaO) obtained by heating 250kg of 95% pu...
Text Solution
|
- Which is the product of the following reaction ?
Text Solution
|
- Which of these carbonyl compounds on reduction with Zn-Hg/HCl will giv...
Text Solution
|
- Which one of the following compound will not show enolisation ?
Text Solution
|