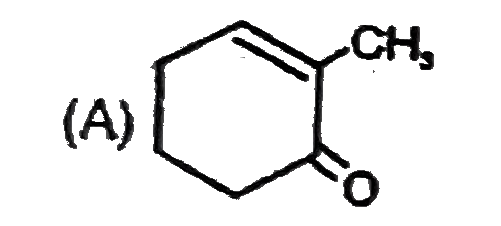
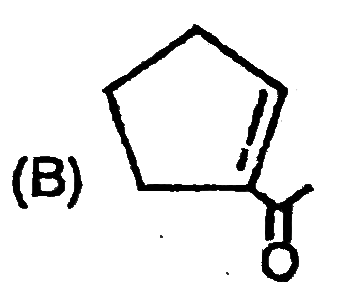
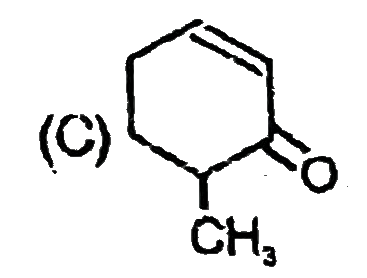
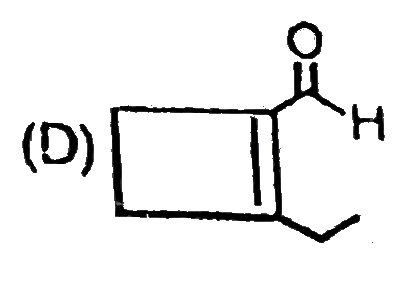
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- CH3CHO+NH2OHoverset(Delta)toPoverset(H^(o+))toQoverset(Br2//KOH)toR(CH...
Text Solution
|
- What is Beckmann Rearrangement ? Explain its mechanism with example .
Text Solution
|
- Intramolecular aldol condensation : The aldol condensation also offe...
Text Solution
|
- What is Aldol condensation reaction ? Explain with example
Text Solution
|
- Intramolecular aldol condensation : The aldol condensation also offe...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 50 per cent ...
Text Solution
|
- Observe the following reactions and its mechanistic steps and intermed...
Text Solution
|
- Observe the following reactions and its mechanistic steps and intermed...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- A gas cylinder was found unattended in a public place. The investigati...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 25 per cent ...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 25 per cent ...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 25 per cent ...
Text Solution
|
- How much amount of CaCO3 in gram having percentage purity 25 per cent ...
Text Solution
|
- The density (in g ml^(−1) ) of a 3.60M sulphuric acid solution having...
Text Solution
|
- Find the concentration of solution in terms of weight percent if 20g o...
Text Solution
|