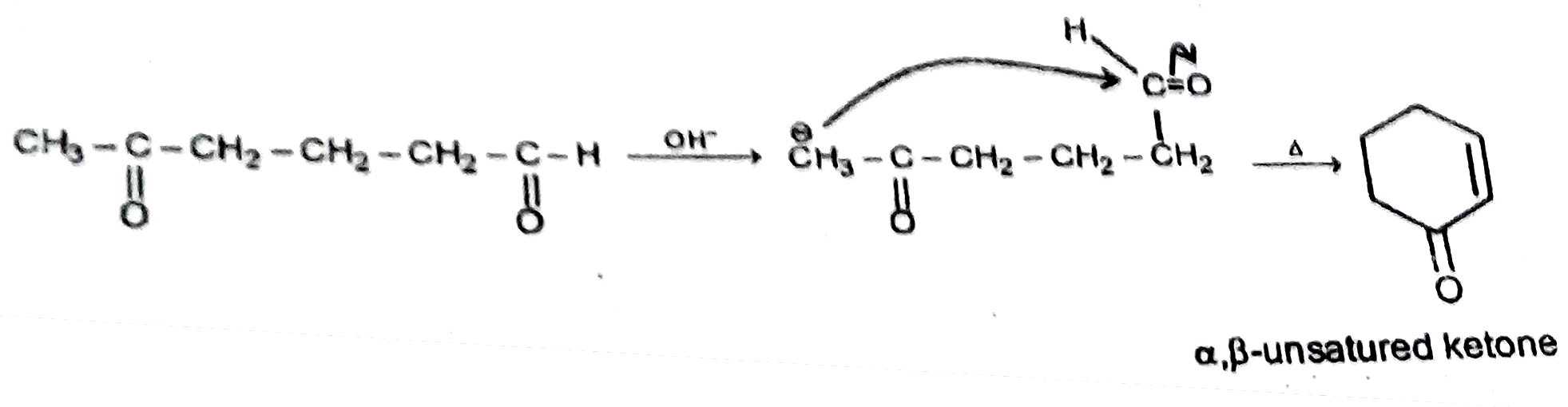
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- NH3 reacts with a gas fumes produced when methanol is passed through C...
Text Solution
|
- State true or false Oxygen is a gas but sulphur a solid.
Text Solution
|
- Why carbon can form stable C=C bond where as silicon can't form Si=Si ...
Text Solution
|
- The only CORRECT combination in which polymerization of hydrolysed pro...
Text Solution
|
- Find the concentration of solution in terms of weight percent if 25g o...
Text Solution
|
- Find the concentration of solution in terms of weight percent if 30g o...
Text Solution
|
- Find the concentration of solution in terms of weight percent if 25g o...
Text Solution
|
- Find the concentration of solution in terms of weight percent if 10g o...
Text Solution
|
- Lactic acid CH3-oversetoverset(OH)(|)CH-oversetoverset(O)(||)C-OH can ...
Text Solution
|
- The product of the following reaction does not show positive test with
Text Solution
|
- Find the concentration of solution in terms of weight percent if 35g o...
Text Solution
|
- Find the concentration of solution in terms of weight percent if 30g o...
Text Solution
|
- In the given reaction , the number of isomeric products formed is - ...
Text Solution
|
- The basicity order of I, II and III is
Text Solution
|
- Complete the following reaction
Text Solution
|
- What will be the most probable product when compound 'X' is treated wi...
Text Solution
|
- Why carbon dioxide can be poured out of gas jar like a liquid?
Text Solution
|
- The correct sequence of reagents for the following conversion is
Text Solution
|
- Which statement is true
Text Solution
|
- X , Y both responds to FeCl3 test and 2,4-DNP test. One is thermodynam...
Text Solution
|