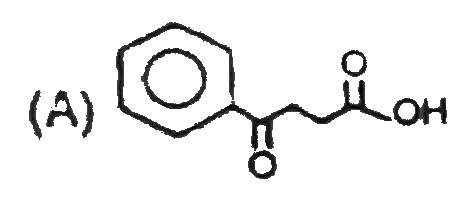
A
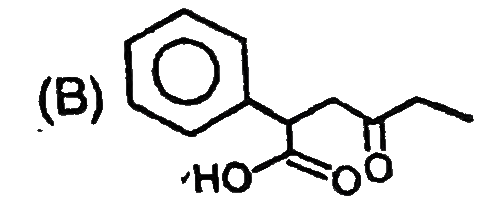
B
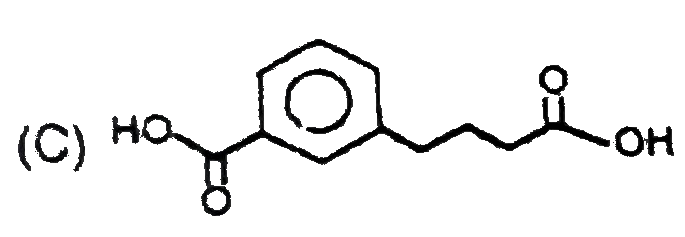
C
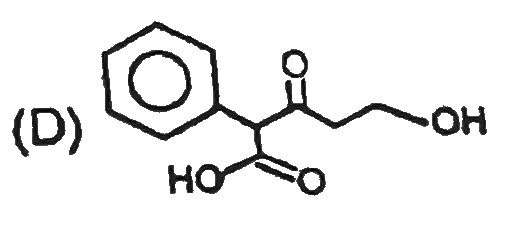
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Complete the following reaction
Text Solution
|
Text Solution
|
- Which of the following undergoes decarboxylation most readily on being...
Text Solution
|
Text Solution
|
- What statement is correct about the following reaction
Text Solution
|
- Identify C is the following sequence of reactions :
Text Solution
|
- Which of the following acid remains unaffected on heating ?
Text Solution
|
- Complete the following reaction
Text Solution
|
- The element whose atomic volume is least is
Text Solution
|
- State true or false The tendency of atom to attract shared pair of e...
Text Solution
|
- State the reaction: Sulphur dioxide gas is passed through an aqueous...
Text Solution
|
- Trans-2-methylcyclohexanol + Acetyl chloride to X X+ NaOH (aq) overs...
Text Solution
|
- How will you seperate propene from propyne ?
Text Solution
|
- In which of the following reactions, the intermediate species acyl nit...
Text Solution
|
- Statement-1 : Electrons releasing group at para position of a migratin...
Text Solution
|
- Statement-1"C-O bond length is shorter in an ester as compared with an...
Text Solution
|
- State true or false halogens are coloured.
Text Solution
|
- State true or false Carbon dioxide is used for making soft drinks.
Text Solution
|
- State true or false SnCl4 is more covalent than SnCl2 .
Text Solution
|
- The element with atomic number 35 belongs to which block?
Text Solution
|