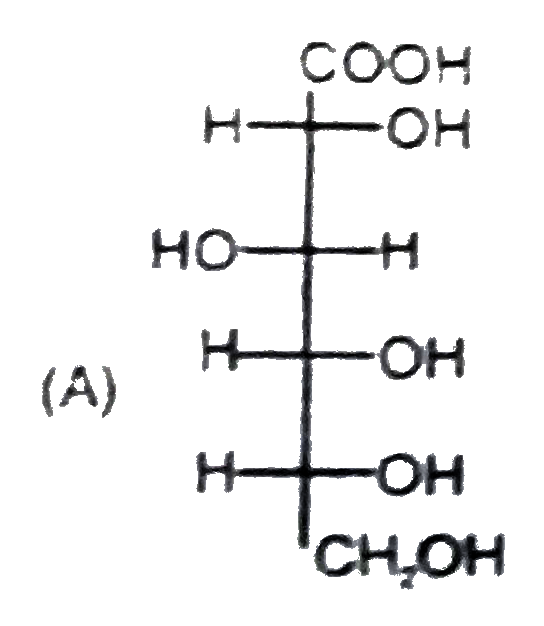
A
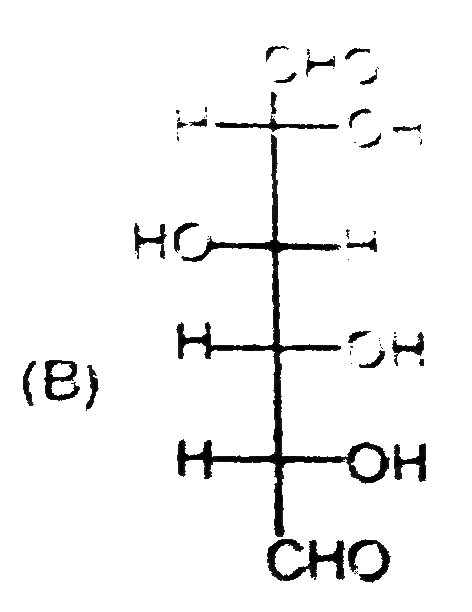
B
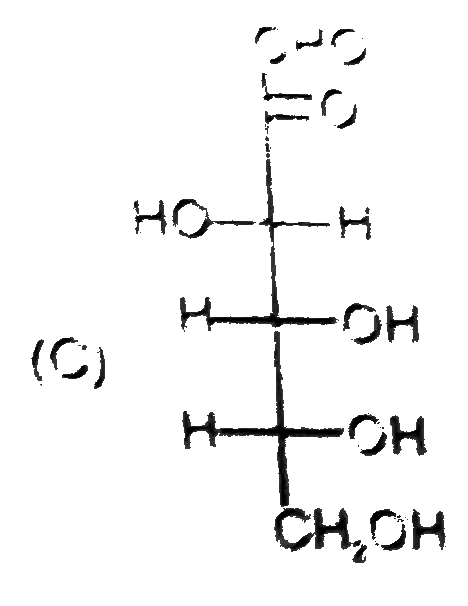
C
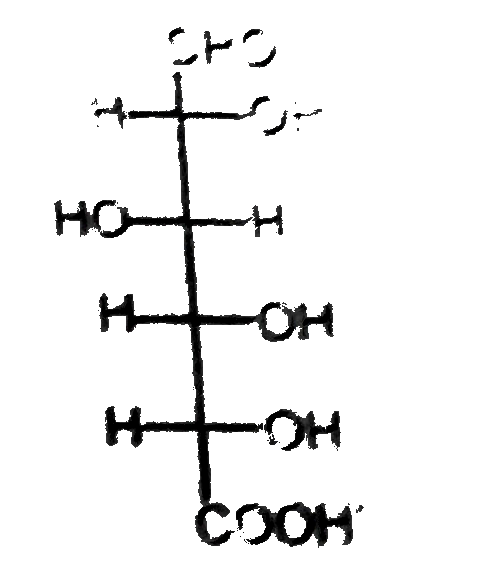
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Which of the following state of carbon dioxide will have lowest densit...
Text Solution
|
- What is known as dead burnt?
Text Solution
|
- D-Glucose upon treatment with bromine-water gives :
Text Solution
|
- is dysentry a water-borne disease :
Text Solution
|
- The above process in which alpha and beta form remain in equilibrium w...
Text Solution
|
- What are interhalogen compounds? Explain with two examples.
Text Solution
|
- Glycoside linkage is :
Text Solution
|
- State true or false Boric acid is prepared from borax by the action...
Text Solution
|
- Nitrous acid (HNO(2)) converts amino acids into hydroxy acids with ret...
Text Solution
|
- Which of the following is a condensation polymer ?
Text Solution
|
- Which two of the following aldohexoses give the same osazone derivativ...
Text Solution
|
- borax is ?
Text Solution
|
- Basic solution of fructose contains :
Text Solution
|
- Which one of the following is non-reducing sugar ?
Text Solution
|
- D - Glucose and D - Mannose are :
Text Solution
|
- How many moles of acetic anhydride (Ac(2)O) is needed to react comple...
Text Solution
|
- Which of the following amino acids is most basic :
Text Solution
|
- Glucose on reduction with Na//Hg and water gives?
Text Solution
|
- What will be product when glycine, is heated -
Text Solution
|
- State true or fasle Borax is a Deca hydrate.
Text Solution
|