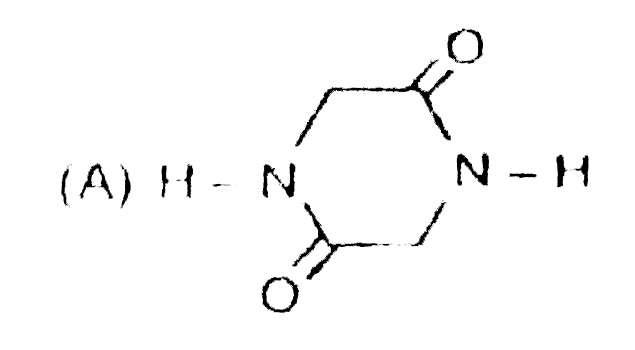
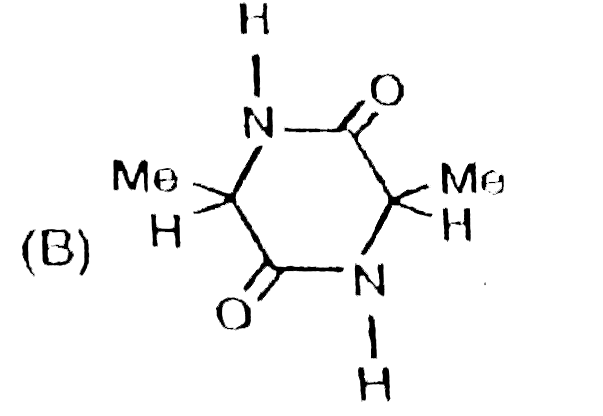
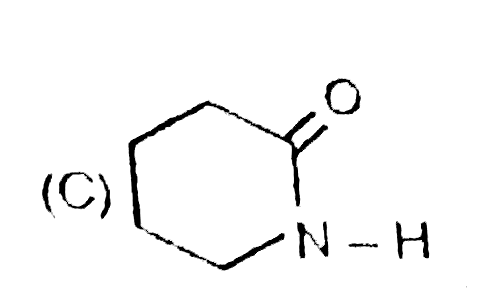
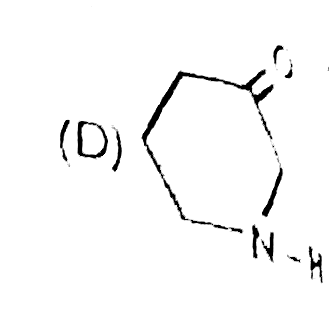
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Which of the following amino acids is most basic :
Text Solution
|
- Glucose on reduction with Na//Hg and water gives?
Text Solution
|
- What will be product when glycine, is heated -
Text Solution
|
- State true or fasle Borax is a Deca hydrate.
Text Solution
|
- Non-metals present in borax are:
Text Solution
|
- State true or false Aqueous solutions of borax acts as a buffer.
Text Solution
|
- Glucosazone is osazone derivativie very similar to that formed from
Text Solution
|
- The correct statements about peptides are
Text Solution
|
- The correct statements about anomers are
Text Solution
|
- State true or false lead does not give the borax bead test.
Text Solution
|
- Statement-1 : Gly-Ala is a structural isomer pf Ala-Gly. Statement-2...
Text Solution
|
- To detect the presence of CO2 water is used.
Text Solution
|
- Statement-1:Pentacetate of glucose does not form oxime on treatment wi...
Text Solution
|
- Statement-1: Glucose contain Straight Chain of 6 carbon atoms. State...
Text Solution
|
- An amino acid is characterized by two pKa values the one corresponding...
Text Solution
|
- An amino acid is characterized by two pKa values the one corresponding...
Text Solution
|
- An amino acid is characterized by two pKa values the one corresponding...
Text Solution
|
- What is true about compound (I)
Text Solution
|
- Which gas is essential constituent of almost all fuel gases?
Text Solution
|
- define water gas?
Text Solution
|